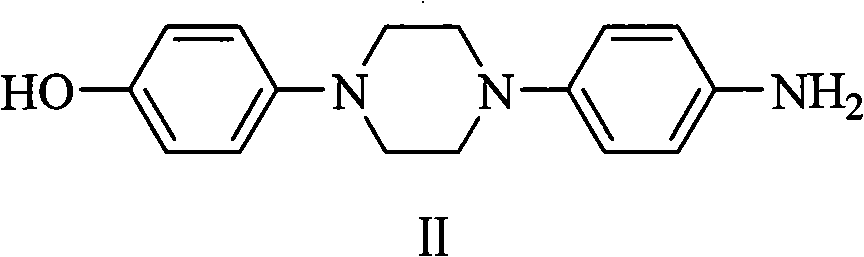
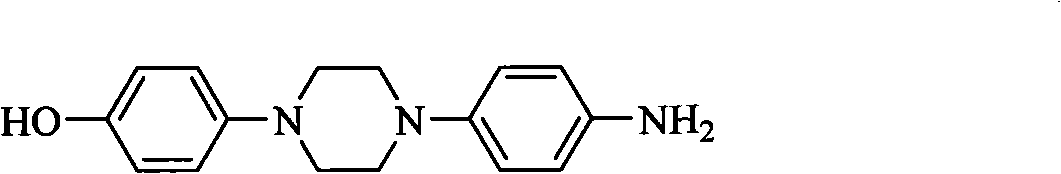
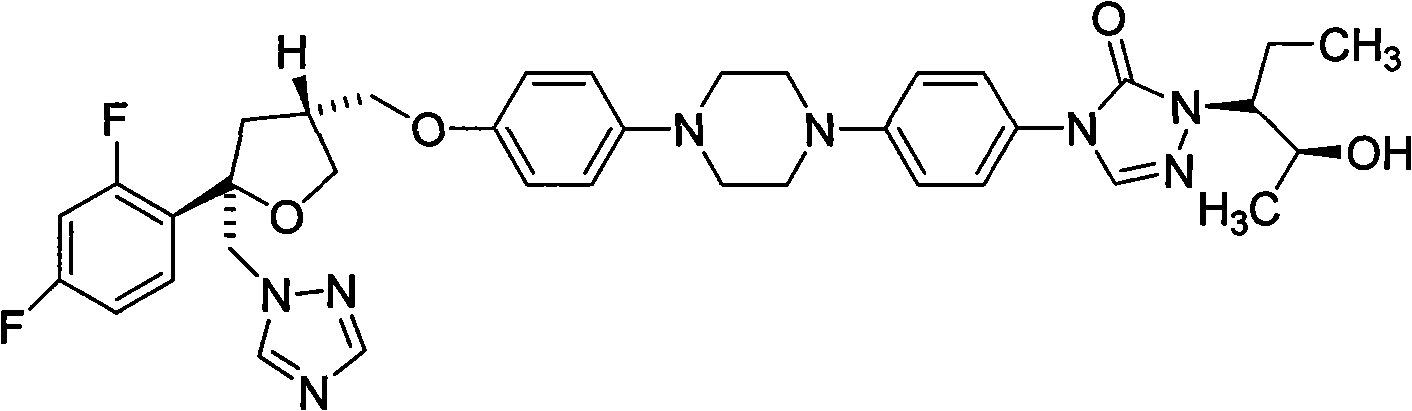
Simple preparation method for posaconazole and piperazine intermediate thereof
A kind of technology of posaconazole and intermediate, which is applied in the field of preparation of 1--4-piperazine, and can solve problems such as being unfavorable for industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine
[0029]
[0030] Add 300 grams of N,N-bis(2-chloroethyl)-4-nitroaniline and 120 grams of p-methoxyaniline into a 3-liter three-necked flask. 1.5 liters of N,N-dimethylformamide was added with stirring, and 20 grams of sodium hydroxide were added in portions. Heated to 100°C for 24 hours. Stop the reaction, stir to cool down, add water to dilute, and then extract three times with 2 liters of chloroform. The organic phase was dried by adding anhydrous potassium carbonate, filtered with suction, and concentrated to remove chloroform. The residue was recrystallized by adding 1,4-dioxane to obtain 202.4 g of the target compound 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine, with a yield of 87%. mp.195.4°C.
Embodiment 2
[0031] Example 2 Synthesis of 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine
[0032] Add 52.5 g of N,N-bis(2-chloroethyl)-4-nitroaniline and 18.4 g of p-methoxyaniline into a 500 ml three-necked flask. 200 ml of toluene was added with stirring, and 2 g of sodium hydroxide were added in portions. Heat to reflux for 24 hours. Stop the reaction, stir and lower the temperature to 50-60°C, add water to dilute, and separate the liquids. The organic phase was washed twice with water, dried by adding anhydrous potassium carbonate,
[0033] Suction filtration, concentration to remove toluene. The residue was recrystallized by adding 1,4-dioxane to obtain 35.2 g of the target compound 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine, with a yield of 88.5%. mp.194.6°C.
Embodiment 3
[0034] Example 3 Synthesis of 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine
[0035] Add 52.5 g of N,N-bis(2-chloroethyl)-4-nitroaniline and 18.4 g of p-methoxyaniline into a 500 ml three-necked flask. 200 ml of N,N-dimethylformamide was added with stirring, and 2 g of sodium hydroxide was added in portions. Heated to 100°C for 24 hours. Stop the reaction, stir and lower the temperature to 50-60°C, add water to dilute, and then extract three times with 450 ml of chloroform. The organic phase was dried by adding anhydrous potassium carbonate, filtered with suction, and concentrated to remove chloroform. The residue was recrystallized by adding 1,4-dioxane to obtain 34.4 g of the target compound 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine, with a yield of 87%. mp.195.1°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com