Netilmicin sulfate lyophiled powder injection and preparation method thereof
A technology of netilmicin sulfate and freeze-dried powder injection is applied in freeze-dried delivery, powder delivery, pharmaceutical formulation and other directions, which can solve the problems of large amount of excipients, complicated preparation methods, hidden safety hazards of injection drugs, etc., and reduce irritation. and toxic and side effects, improve drug stability, improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
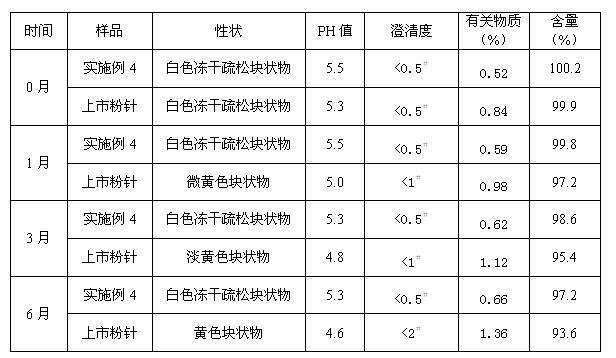
Examples
Embodiment 1
[0021] Recipe: Netilmicin Sulfate 100g
[0022] Bletilla striata polysaccharide 75g
[0024] Makes 1000 bottles
[0025] Preparation:
[0026] (1) Preparation: Routinely handle the pipes and containers used for dosing, and then wash them with water for injection; the vials and rubber stoppers should be roughly washed and then rinsed, then dried, sterilized, and cooled for later use.
[0027] (2) Liquid preparation: first weigh the prescribed amount of Bletilla striata polysaccharide, add 800ml of water for injection to make a 40% solution, add netilmicin sulfate and 1200ml of water for injection, stir until the whole amount is dissolved.
[0028] (3) Decolorization: add medicinal charcoal with 0.1% of the total volume of the solution, stir and absorb at room temperature for more than 30 minutes to obtain a suspension of netilmicin sulfate, take a sample, measure the pH value of the liquid, add sodium carbonate to adjust the pH to...
Embodiment 2
[0033] Recipe: Netilmicin Sulfate 100g
[0034] β-cyclodextrin 85g
[0035] Sodium bicarbonate 0.6g
[0036] Makes 1000 bottles
[0037] Preparation:
[0038] (1) Preparation: Routinely handle the pipes and containers used for dosing, and then wash them with water for injection; the vials and rubber stoppers should be roughly washed and then rinsed, then dried, sterilized, and cooled for later use.
[0039] (2) Dosing: first weigh the prescribed amount of β-cyclodextrin, add 800ml of water for injection to make a 40% solution, add netilmicin sulfate and 1200ml of water for injection, stir until the whole amount is dissolved.
[0040] (3) Decolorization: add 0.1% of the total volume of medicinal charcoal in the solution, stir and absorb at room temperature for more than 30 minutes to obtain a suspension of netilmicin sulfate, take a sample, measure the pH value of the liquid, add sodium bicarbonate to adjust the pH to 5.0 , the measured content was 48.7mg / ml....
Embodiment 3
[0045] Recipe: Netilmicin Sulfate 100g
[0046] Urea 90g
[0047] Ammonia 0.8g
[0048] Makes 1000 bottles
[0049] Preparation:
[0050] (1) Preparation: Routinely handle the pipes and containers used for dosing, and then wash them with water for injection; the vials and rubber stoppers should be roughly washed and then rinsed, then dried, sterilized, and cooled for later use.
[0051] (2) Dosing: first weigh the prescribed amount of urea, add 900ml of water for injection to make a 45% solution, add netilmicin sulfate and 1100ml of water for injection, stir until the full amount is dissolved.
[0052] (3) Decolorization: Add 0.3% medicinal charcoal in the total volume of the solution, stir and adsorb at room temperature for more than 30 minutes to obtain a suspension of netilmicin sulfate, take a sample, measure the pH value of the liquid, add ammonia water to adjust the pH to 5.3, and measure The content is 49.2mg / ml.
[0053] (4) Filtration and subpackagi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com