Preparation method of moxifloxacin hydrochloride
A technology for moxifloxacin hydrochloride and ethyl quinoline carboxylate, which is applied in the field of pharmaceutical production, can solve the problems that the quality of moxifloxacin hydrochloride cannot be guaranteed, is not suitable for industrial production, and is difficult to control the quality of crystallization finished products, and improves the preparation efficiency. And the quality of the finished product, improve the reaction speed and reaction efficiency, optimize the effect of the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
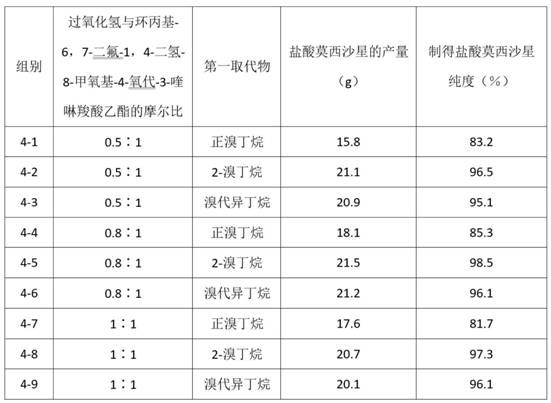
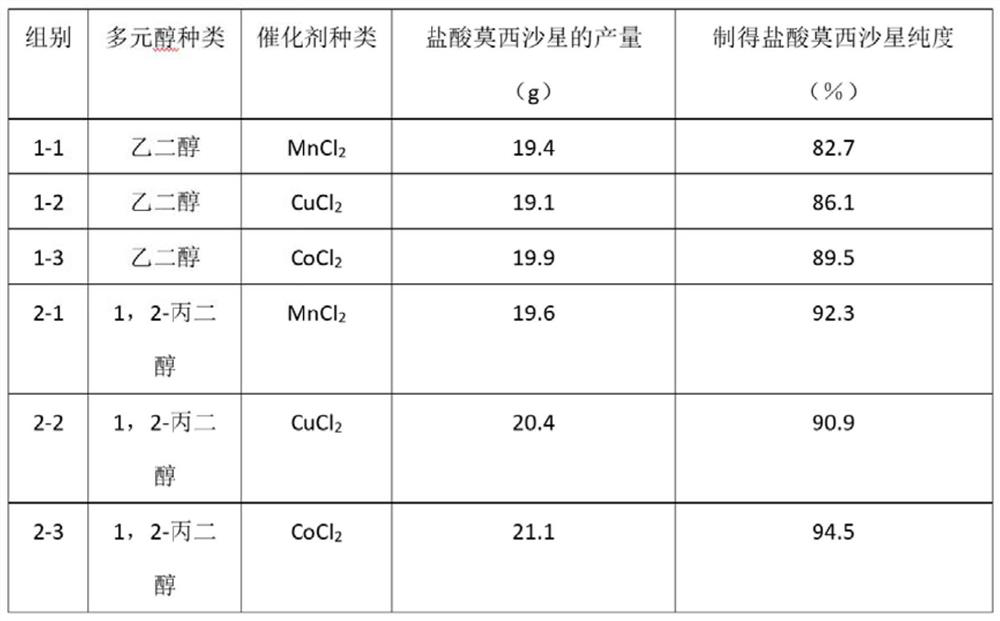
[0070] Group 1-1
[0071] A preparation method for moxifloxacin hydrochloride, comprising the following steps:
[0072] Under the protection of nitrogen, add to the reactor, 3.3g ethylene glycol, 0.8gMnCl 2 , 160mL ethanol and 16.2g 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxyl-4-oxo-3-quinolinecarboxylic acid ethyl ester, at a temperature of 40°C 274g of 2-bromobutane was added to the reaction kettle, sodium hydroxide was added, and the pH was adjusted to 8, and the reaction kettle was continued to be heated at 54°C for 4h; under the protection of nitrogen, Sodium hydroxide was added in the reaction kettle, and the pH was adjusted to be 8, and 160mL acetone and 6.3g (S, S)-2,8-diazabicyclo[4.3.0]nonane were added in the reaction kettle, at 80 Under the temperature of ℃, after reacting for 4h, add 2.8g of 30% hydrogen peroxide solution in the reactor, control the reaction temperature to be 70℃, and continue the heating reaction for 5h; after the reaction is completed, ex...
Embodiment 2
[0080] Group 2-1
[0081] A preparation method for moxifloxacin hydrochloride, comprising the following steps:
[0082] Under the protection of nitrogen, add to the reactor, 3.8g1,2-propanediol, 0.8gMnCl 2, 160mL ethanol and 16.2g 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxyl-4-oxo-3-quinolinecarboxylic acid ethyl ester, at a temperature of 40°C 274g of 2-bromobutane was added to the reaction kettle, sodium hydroxide was added, and the pH was adjusted to 8, and the reaction kettle was continued to be heated at 54°C for 4h; under the protection of nitrogen, Sodium hydroxide was added in the reaction kettle, and the pH was adjusted to be 8, and 160mL acetone and 6.3g (S, S)-2,8-diazabicyclo[4.3.0]nonane were added in the reaction kettle, at 80 Under the temperature of ℃, after reacting for 4h, add 2.8g of 30% hydrogen peroxide solution in the reactor, control the reaction temperature to be 70℃, and continue the heating reaction for 5h; after the reaction is completed, expo...
Embodiment 3
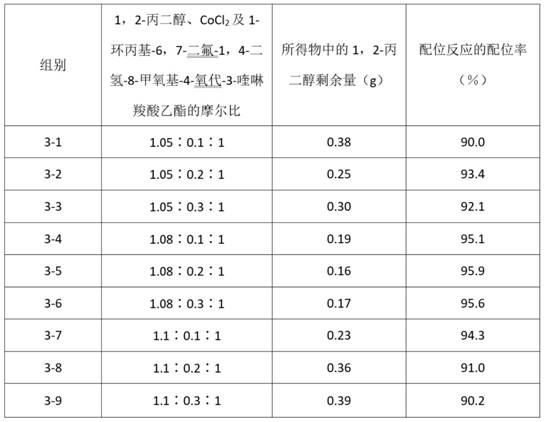
[0092] Group 3-1
[0093] Coordination reaction, under the protection of nitrogen, add 3.8g1,2-propanediol, 0.7gCoCl to the reaction kettle 2 , 160mL ethanol and 16.2g 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxyl-4-oxo-3-quinolinecarboxylic acid ethyl ester, at a temperature of 40°C , reacted for 5 h, and measured the content of 1,2-propanediol in the coordination reaction product by using HPLC, and calculated the coordination ratio of the coordination reaction according to the remaining amount of 1,2-propanediol.
[0094] Group 3-2
[0095] Coordination reaction, under the protection of nitrogen, add 3.8g1,2-propanediol, 1.4gCoCl to the reaction kettle 2 , 160mL ethanol and 16.2g 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxyl-4-oxo-3-quinolinecarboxylic acid ethyl ester, at a temperature of 40°C , reacted for 5 h, and measured the content of 1,2-propanediol in the coordination reaction product by using HPLC, and calculated the coordination ratio of the coordination...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com