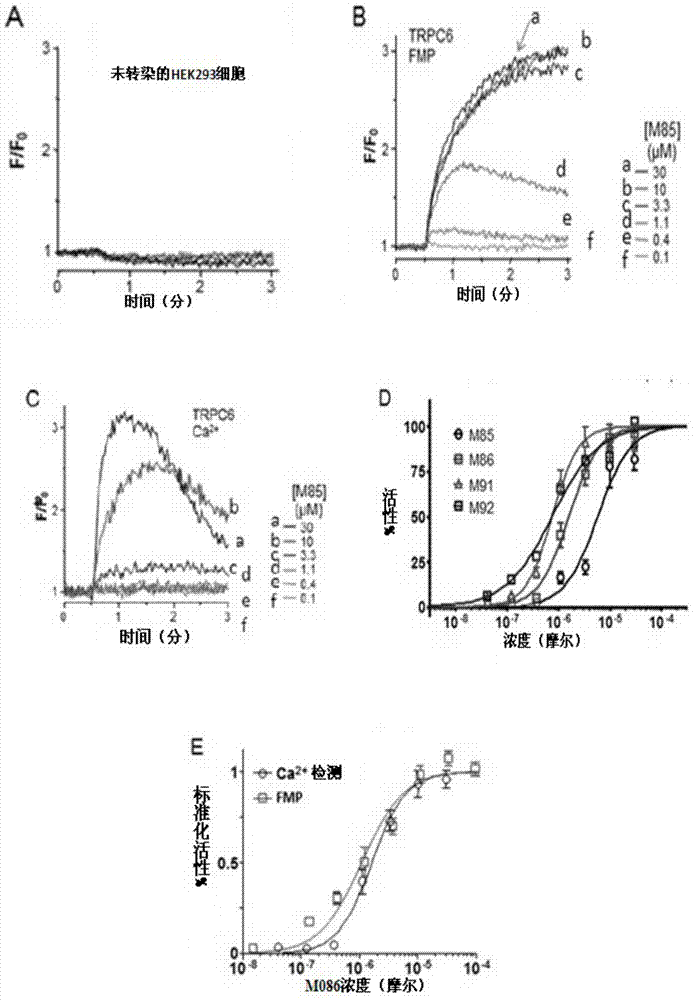
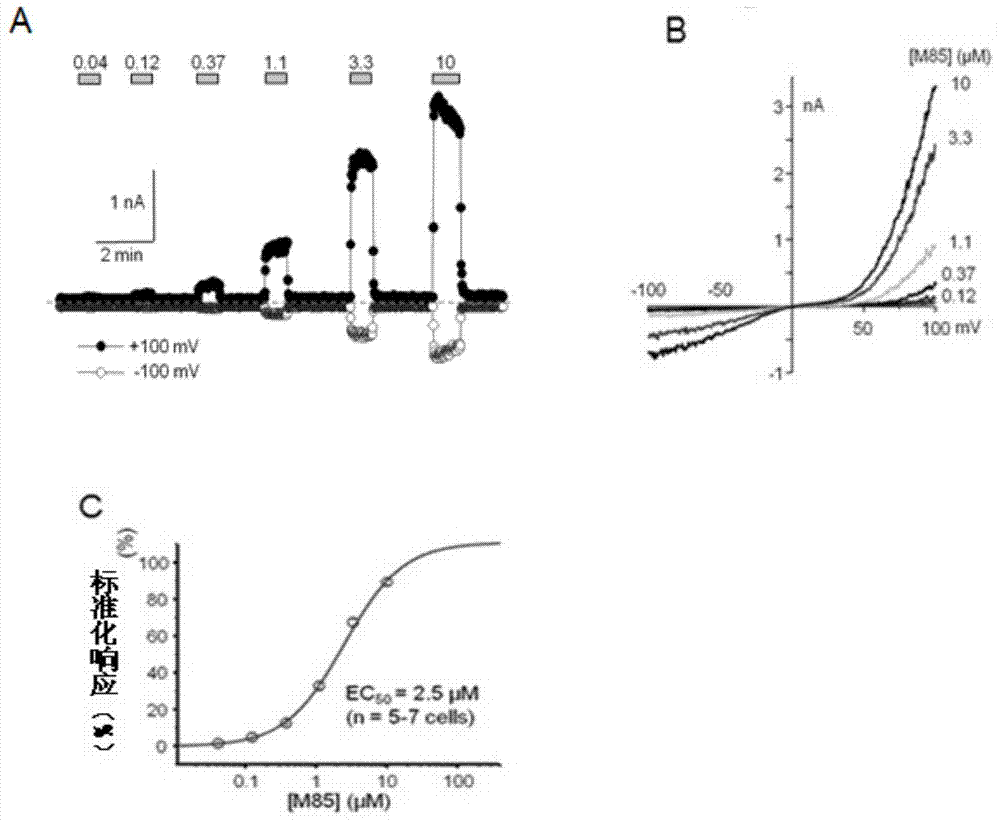
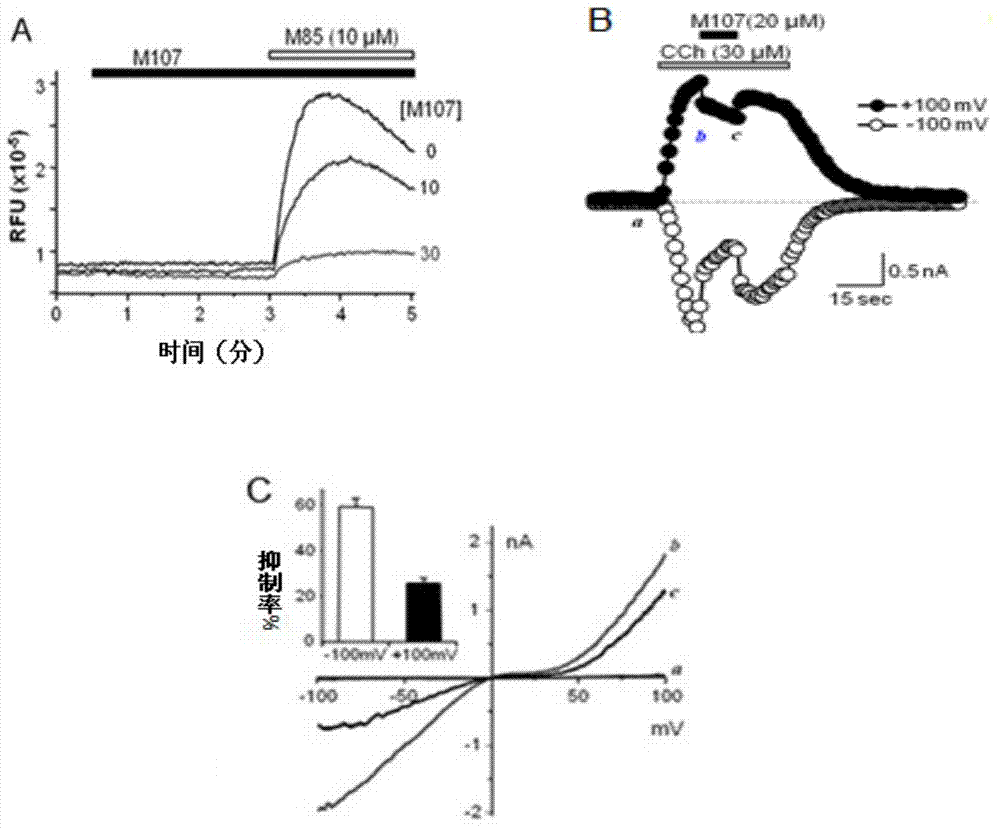
Pyrazolopyrimidine compound and pharmaceutical composition thereof as well as pharmaceutical application of pyrazolopyrimidine compound
A technology for pyrazolopyrimidines and compounds, applied in the field of pyrazolopyrimidine compounds and their pharmaceutical compositions and their preparation, capable of solving problems such as inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Compound M106, wherein R 1 is hydrogen, R 2 is 4-fluorophenyl, R 3 Yes where n=0, X is C, Y is N, R 6 is tert-butoxycarbonyl, R 4 The preparation process of the compound M106 that is a hydroxyl group is as follows:
[0049]
[0050] Step a: Synthesis of intermediate 1: Take 4.12g (32mmol) of the starting material 4-piperidinecarboxylic acid and add it to a 100mL round bottom flask, add water 32mL, K 2 CO 3 6.78g (64mmol), cool down to 0°C, take 6.98g (32mmol) of di-tert-butyl dicarbonate in anhydrous condition, dissolve it in freshly distilled THF 5mL, add dropwise to a 100mL round-bottomed flask, after the dropwise addition, raise to room temperature and stir for 4h . After the reaction was completed, the THF was removed by rotary evaporation, and the 2 Wash the aqueous phase with O (20mL×2), adjust the pH of the aqueous phase to 2 with 6M HCl, then extract with EA (30mL×3), combine the EA phases, and wash with anhydrous MgSO 4 Dried, filtered and spin-dri...
Embodiment 2
[0058] Compound M086, R 1 is hydrogen, R 2 is 4-fluorophenyl, R 3 Yes where n=0, X is H, Y is N, R 6 is benzyloxycarbonyl, R 4 The preparation process of the compound M086 that is a hydroxyl group is as follows:
[0059]
[0060] Step a: Synthesis of Intermediate 1: Take 6.46g (50mmol) of the starting material 4-piperidinecarboxylic acid and add it to a 100°C round bottom flask, add 4.4g (110mmol) of NaOH, add 25mL of water, cool down to 0°C, and add dropwise Benzyl chloroformate 9.38g (5.5mmol), after the dropwise addition was completed, it was raised to room temperature and stirred for 4h. After the reaction, use Et 2 Wash the reaction solution with O (20mL×2), adjust the pH of the aqueous phase to 2 with 6M HCl, then extract with EA (30mL×3), combine the EA phases, and wash with anhydrous MgSO 4 Dry, filter and spin-dry the obtained product Intermediate 1 as a white solid. Yield 94.9%.
[0061] Step b: Synthesis of Intermediate 2: Under anhydrous conditions, ta...
Embodiment 3
[0068] Compound M085, R 1 is hydrogen, R 2 is 4-fluorophenyl, R 3 Yes where n=0, X is H, Y is N, R 6 is ethoxycarbonyl, R 4 The preparation process of the compound M085 which is a hydroxyl group is as follows
[0069]
[0070] Step a: Synthesis of Intermediate 1: Take 5.16 g (40 mmol) of the starting material 4-piperidinecarboxylic acid and add it to a 100-degree round bottom flask, add Na 2 CO 3 Add 40mL of water to 5.04g (48mmol), dissolve 5.13g (48mmol) in 40mL of tetrahydrofuran, add dropwise to a round bottom flask, and stir at room temperature for 2h. At the end of the reaction, tetrahydrofuran was removed by rotary evaporation, 60 mL of water was added, the pH was adjusted to 2 with 2M HCl, and then extracted with EA (30 mL×3), the EA phases were combined and washed with anhydrous MgSO 4 Dry, filter and spin-dry the obtained product Intermediate 1 as a white solid. Yield 91.9%.
[0071] Step b: Synthesis of Intermediate 2: Under anhydrous conditions, take I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com