A kind of preparation method of dry powder inhaler
A technology for dry powder inhaler and equipment, which is applied in the field of preparation of dry powder inhaler, can solve problems such as difficulty in controlling and reduce drug efficacy, and achieve the effects of good content uniformity, excellent inhalation characteristics, and suitable aerodynamic fine particle fraction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 - Preparation of micronized active ingredient
[0043] Aclidinium bromide is micronized in a jet mill, the pulverization pressure is 0.6Mpa, and the particle size D50 of the obtained product reaches 2.8 μm, and D10>1 μm.
[0044] The umeclidinium bromide is respectively micronized in a jet mill, the pulverization pressure is 0.4Mpa, and the particle size D50 of the obtained product reaches 2-3 μm, and D10>1 μm.
[0045] The glycopyrronium bromide is respectively micronized in a jet mill, the pulverization pressure is 0.4Mpa, and the particle size D50 of the obtained product reaches 2-3 μm, and D10>1 μm.
[0046] Micronize formoterol fumarate dihydrate and formoterol fumarate in a jet mill respectively, the pulverization pressure is 0.6Mpa, the particle size D50 of the obtained product reaches 2-3μm, and D10>1μm .
[0047] Indacaterol fumarate is micronized in a jet mill respectively, the pulverization pressure is 0.8Mpa, and the particle size D50 of the ob...
Embodiment 2
[0051] The preparation of embodiment 2-aclidinium bromide dry powder inhalation
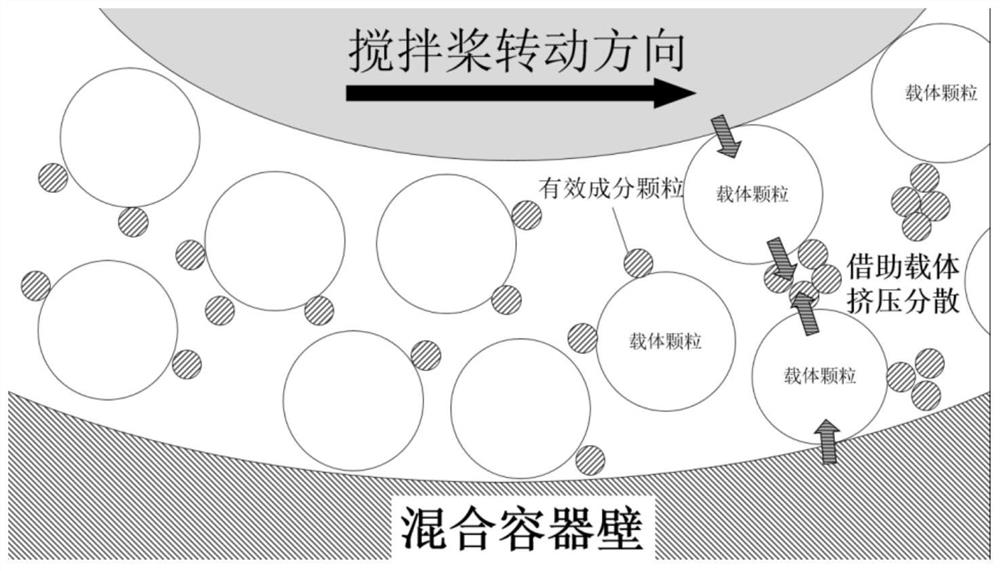
[0052] The aclidinium bromide of lactose monohydrate and the micronization in the following table is mixed in a three-dimensional mixer for 10min, transferred to a high-speed mixer, and an appropriate paddle is selected so that the gap between the paddle and the container wall is 0.5mm, reverse the stirring paddle, mix with the paddle linear speed of 5m / s for 10min, and let stand properly to prepare aclidinium bromide dry powder inhaler.
[0053]
Embodiment 3
[0054] The preparation of embodiment 3-aclidinium bromide dry powder inhalation
[0055] The aclidinium bromide of lactose monohydrate and the micronization in the following table is mixed in a three-dimensional mixer for 10min, transferred to a high-speed mixer, and an appropriate paddle is selected so that the gap between the paddle and the container wall is 1.0mm, reverse the stirring paddle, mix with the paddle linear speed of 8m / s for 10min, and let stand properly to prepare aclidinium bromide dry powder inhaler.
[0056]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com