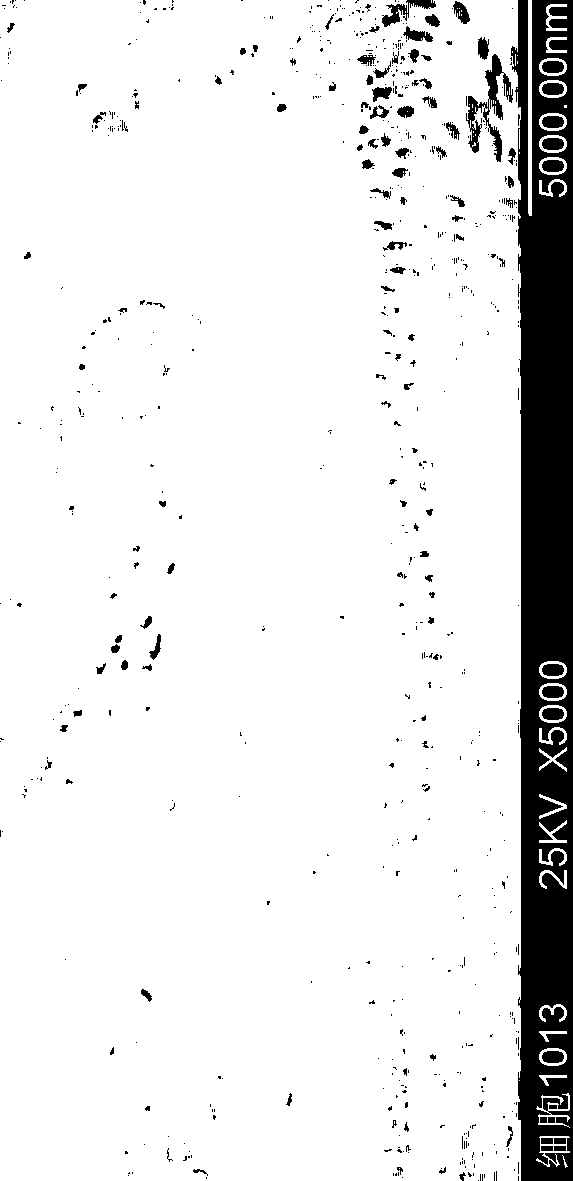
Cyclosporin A pulmonary inhaled macroporous microspheres and preparation method thereof
A technology of macroporous microspheres and cyclosporine, which is applied in the fields of cyclic peptide components, respiratory system diseases, inactive components of polymer compounds, etc. problems such as large differences, to achieve the effect of good biocompatibility, good biocompatibility and smooth surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A cyclosporin A macroporous microsphere for pulmonary inhalation, prepared from the following components:
[0040] Cyclosporin A 1g;
[0041] Chitosan derivative 1g;
[0042] β-cyclodextrin 0.5g;
[0043] Porogen 0.5g;
[0044] The chitosan quaternary ammonium salt is a high molecular weight chitosan quaternary ammonium salt with a molecular weight of 1360kDa, and the substitution degree is 90%.
[0045] The porogen is ammonium bicarbonate.
[0046] The preparation method of the cyclosporin A lung inhalation macroporous microspheres comprises the following steps:
[0047] (1) Prepare a mixed solution of each component according to the above ratio requirements, weigh vacuum-dried β-cyclodextrin, dissolve it in 200 mL of HTCC (molecular weight: 1.3 million) distilled water, and dissolve ammonium bicarbonate in the sample solution ;
[0048] (2) After filtering through a 0.45 μm microporous membrane, adjust the mass concentration of the mixed filtrate to 0.5-1.5%;
...
Embodiment 2
[0053] A cyclosporin A macroporous microsphere for pulmonary inhalation, prepared from the following components:
[0054] Cyclosporine A 2g;
[0055] Chitosan derivative 5 g;
[0056] β-cyclodextrin 2.5 g;
[0057] Porogen 2.5 g;
[0058] Described chitosan derivative is chitosan quaternary ammonium salt;
[0059] The chitosan quaternary ammonium salt is a high molecular weight chitosan quaternary ammonium salt with a molecular weight of 1100kDa, and the substitution degree is 90%.
[0060] The porogen is propylene glycol block polyether (F68).
[0061] The preparation method of the cyclosporin A lung inhalation macroporous microspheres comprises the following steps:
[0062] (1) Prepare a mixed solution of each component according to the above ratio requirements, weigh vacuum-dried β-cyclodextrin, dissolve it in 200 mL of HTCC (molecular weight: 1.3 million) distilled water, and dissolve F68 in the above-prepared mixed solution. in solution;
[0063] (2) After filtering ...
Embodiment 3
[0068] A cyclosporin A macroporous microsphere for pulmonary inhalation, prepared from the following components:
[0069] Cyclosporin A 3g;
[0070] Chitosan derivative 10g;
[0071] β-cyclodextrin derivative 5g;
[0072] Porogen 5g;
[0073] Described chitosan derivative is chitosan quaternary ammonium salt;
[0074] The chitosan quaternary ammonium salt is a chitosan quaternary ammonium salt with a molecular weight of 450kDa and a substitution degree of 85%.
[0075] The β-cyclodextrin derivative is 2-hydroxypropyl-β-cyclodextrin (Hp-β-CD);
[0076] The porogen is polyethylene glycol.
[0077] The preparation method of the cyclosporin A lung inhalation macroporous microspheres comprises the following steps:
[0078] (1) Prepare the mixed solution of each component according to the above ratio requirements, weigh the vacuum-dried Hp-β-CD, dissolve it in 200 mL of HTCC (molecular weight: 1.3 million) distilled water, and dissolve the polyethylene glycol in the above prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com