Integrated device and method for improving pulmonary inhalation medication through lactose micropowder pre-deposition
A lactose micropowder and pre-deposition technology, applied in inhalers, pharmaceutical formulations, drug delivery, etc., can solve the problems of unknown safety and few cases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
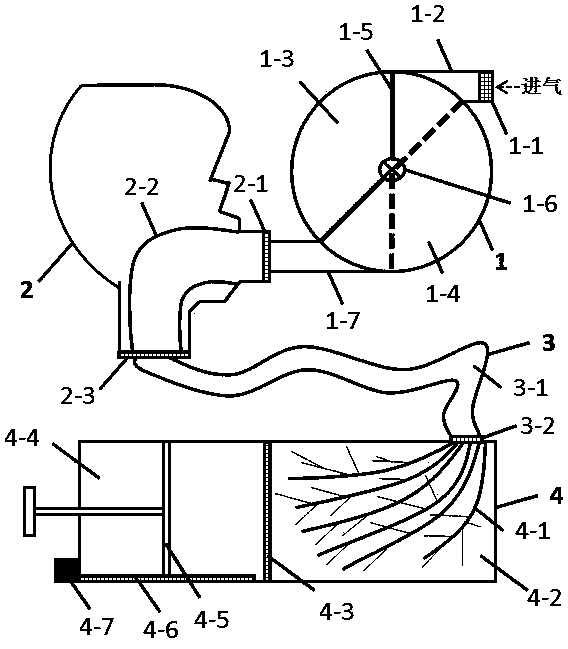
[0029] like figure 1 (Schematic diagram of the device integration for improving pulmonary inhalation by pre-deposition of lactose micropowder) As shown, the device integration for improving pulmonary inhalation by pre-deposition of lactose micropowder mainly includes inhaler (1), head and throat (2) , Breathing pipeline (3) and lung model equipment (4), the air outlet of the inhaler (1) is connected with the air inlet of the head and throat (2), and the air outlet of the head and throat (2) is connected with the air inlet of the breathing pipeline (3) The air outlet of the respiratory pipeline (3) is connected with the air inlet of the lung model equipment (4); the inhaler (1) mainly includes a filter tip (1-1), an air inlet of the inhaler (1-2) , powder compartment (1-3), lactose micropowder compartment (1-4), V-shaped compartment switching baffle (1-5), compartment switching knob (1-6) and inhaler air outlet (1- 7), the head and throat (2) mainly includes the head and throa...
Embodiment 2
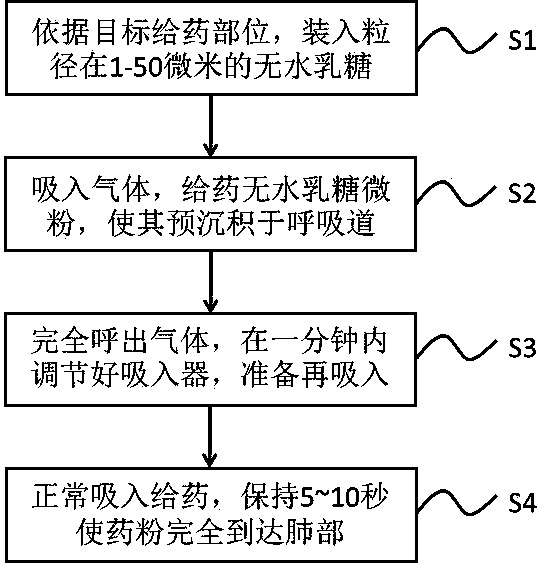
[0042] According to the device integration and method for improving lung inhalation medication through pre-deposition of lactose micropowder, this embodiment provides a specific clinical drug delivery procedure, including:
[0043] S1: For the target administration site is the respiratory tract and lungs, load 20 mg of flower-shaped lactose with a particle size of 10-50 microns (the material has a nitrogen adsorption surface area of 20-30m 2 / g);
[0044] S2: Inhalation of gas, administration of anhydrous lactose micropowder to make it pre-deposited in the respiratory tract;
[0045] S3: Exhale completely, adjust the inhaler within one minute, and prepare for reinhalation;
[0046] S4: Administer tiotropium bromide powder normally by inhaling it, and keep it for 5-10 seconds so that the powder reaches the lungs completely.
[0047] use figure 1 The equipment shown is simulated, first inhaling anhydrous lactose micropowder, making it settle in the wet pipeline, then inhali...
Embodiment 3
[0049] According to the device integration and method for improving lung inhalation medication through pre-deposition of lactose micropowder, this embodiment provides a specific clinical drug delivery procedure, including:
[0050] S1: For the target drug administration site is the lungs, load 100 mg of flower-shaped lactose with a particle size of 5-10 microns (the nitrogen adsorption surface area of this material is 30-40m 2 / g);
[0051] S2: Inhalation of gas, administration of anhydrous lactose micropowder to make it pre-deposited in the respiratory tract;
[0052] S3: Exhale completely, adjust the inhaler within one minute, and prepare for reinhalation;
[0053] S4: Administer budesonide powder by normal inhalation, and keep for 5-10 seconds so that the powder reaches the lungs completely.
[0054] use figure 1 The equipment shown is simulated, first inhaling anhydrous lactose micropowder, making it settle in the wet pipeline, then inhaling the drug powder, and measu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com