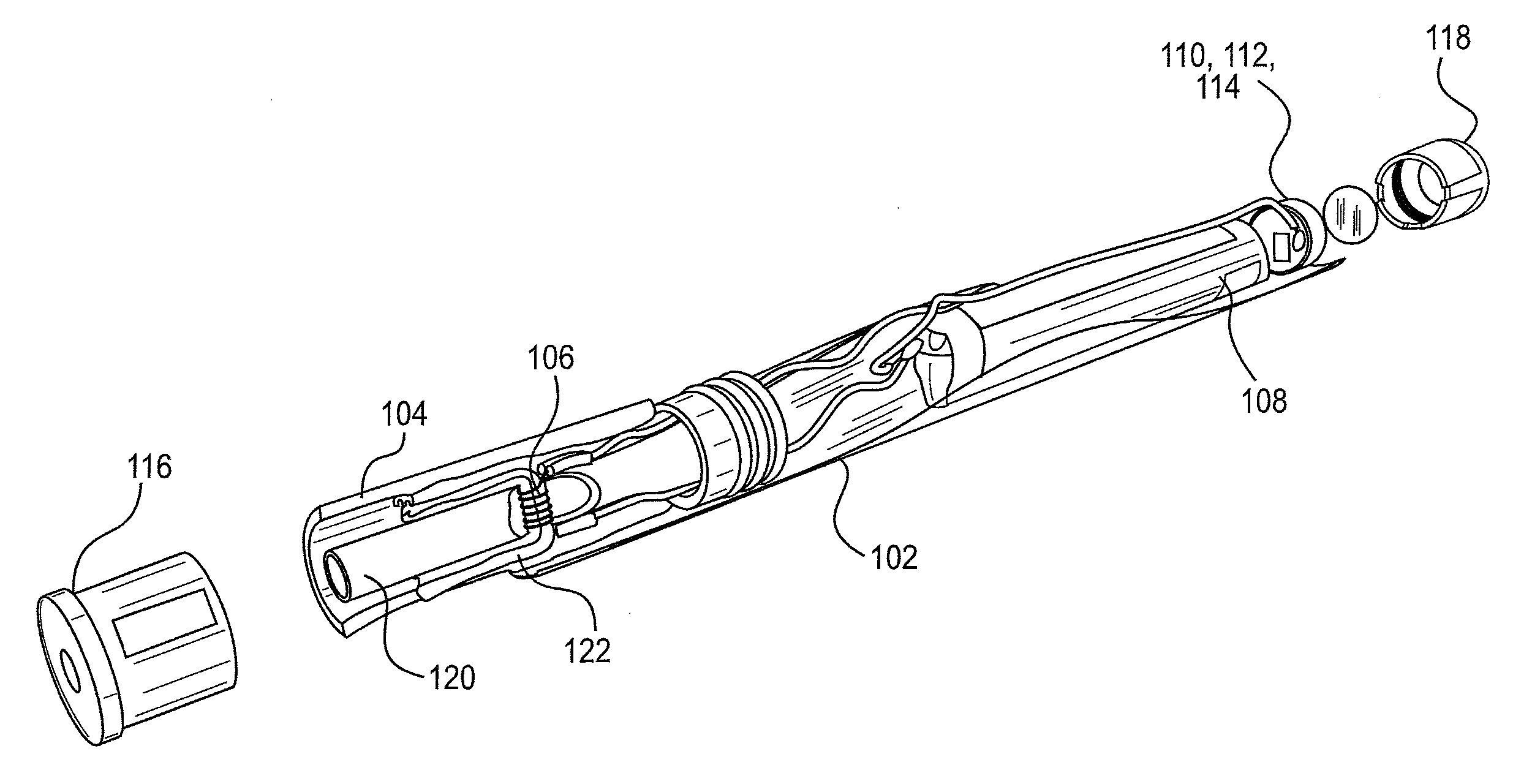
Patents
Literature
32 results about "Lung deposition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
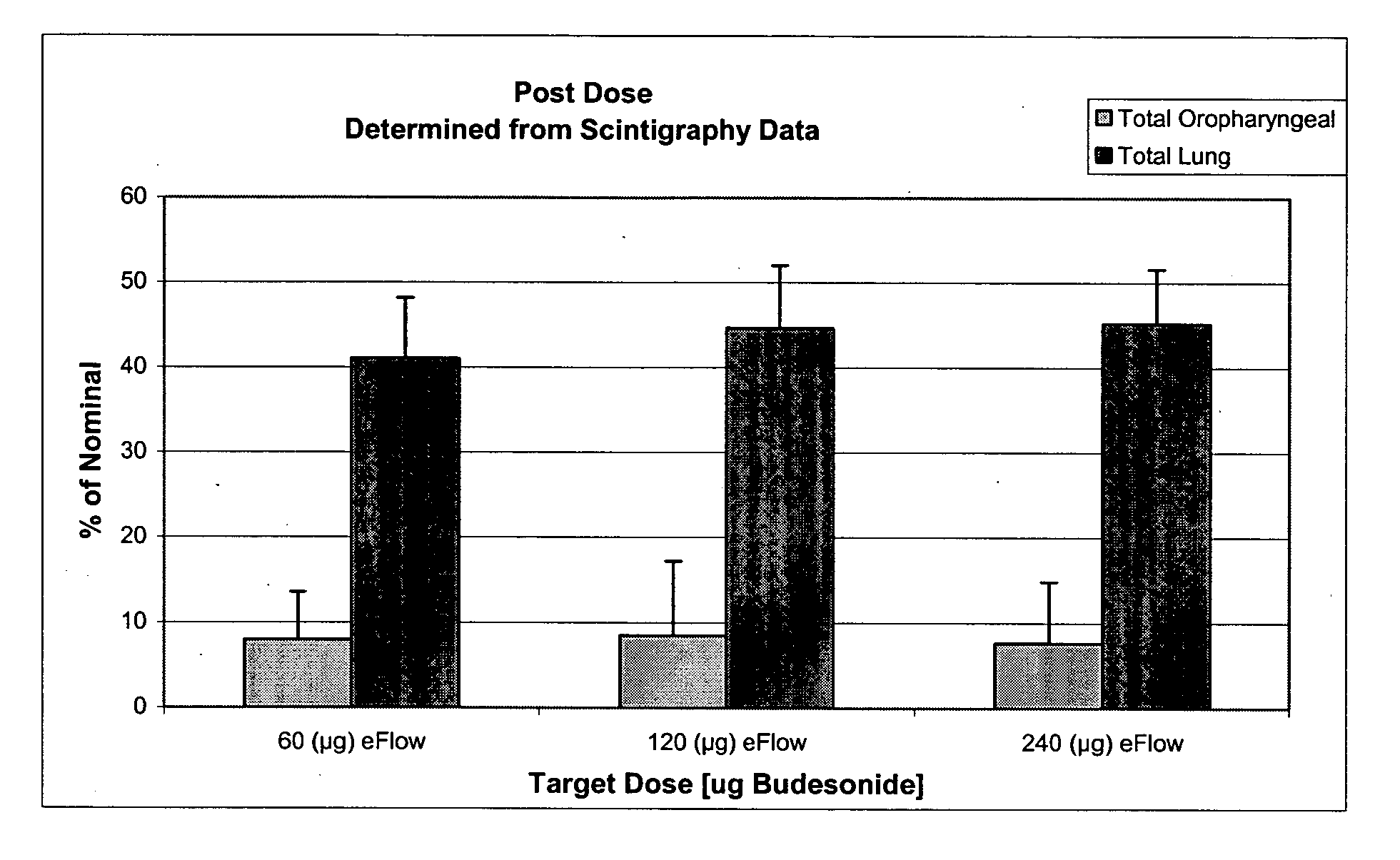
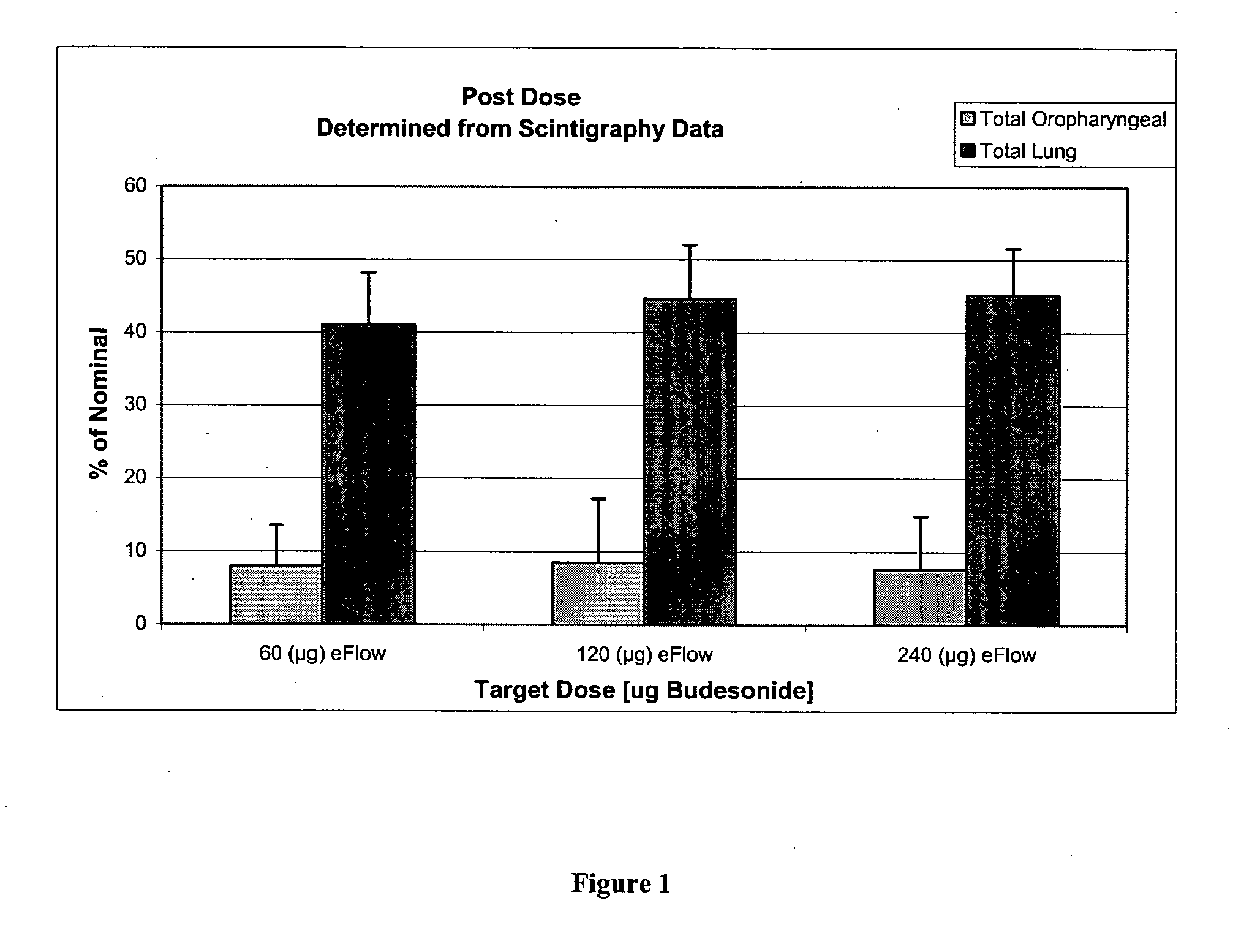
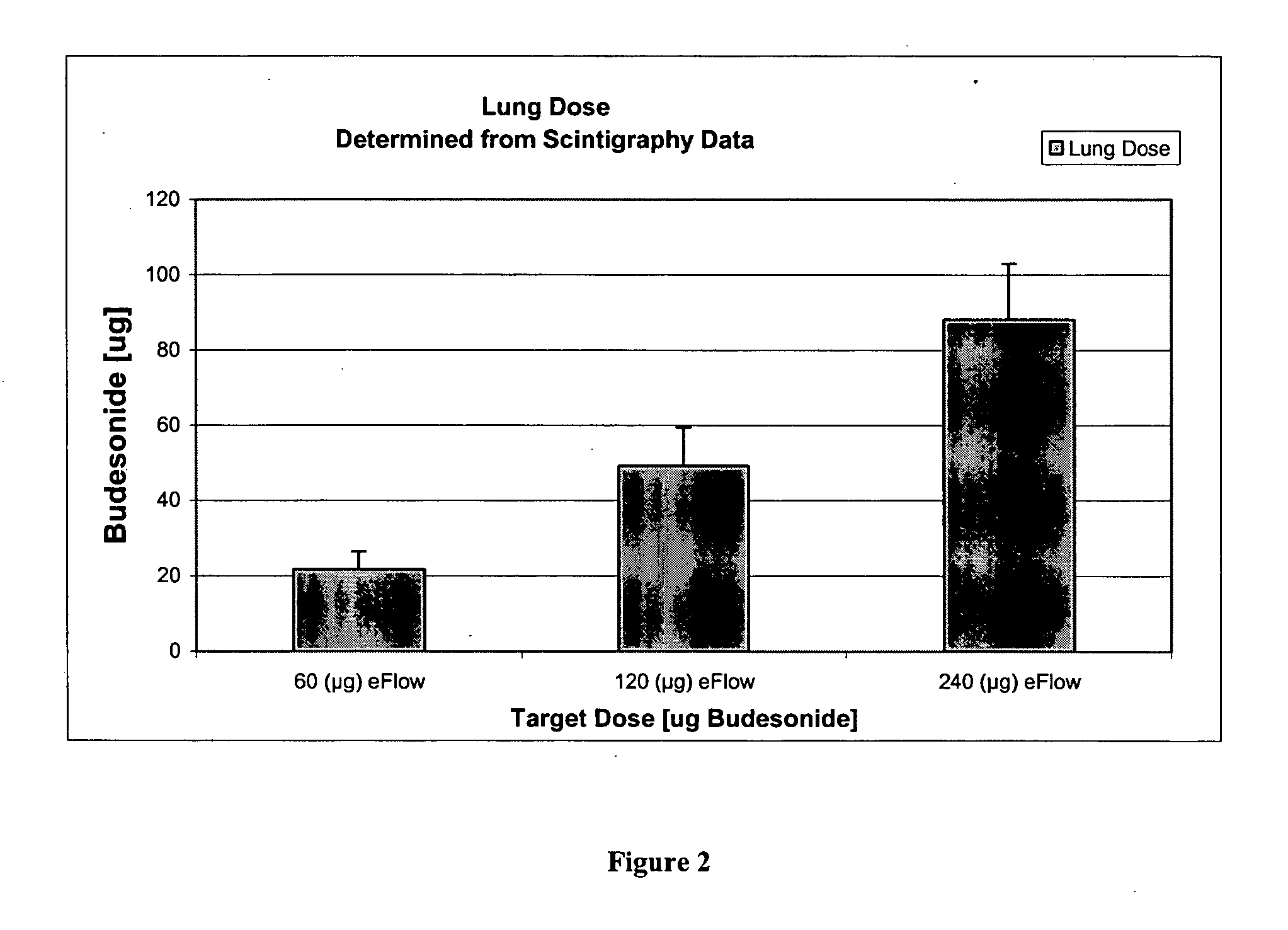
Each lobe of the lung received a different amount of deposition since deposition correlates with lobar volume. This is evident by comparing mean deposition fractions in different lobes of the lung (Fig. 3). Basically, the left lung received higher deposition than each lobe of the right lung.
Compositions, devices, and methods for nicotine aerosol delivery
ActiveUS20140345635A1Reduce degradationConstant efficiencyTobacco treatmentTobacco devicesSolventElectron
The present disclosure generally relates to compositions, and related devices and methods, useful in vaporizing devices such as electronic cigarettes. The composition may comprise nicotine, at least one solvent, and at least one ion pairing agent, and may be vaporized to form a condensation aerosol, wherein inhalation of the aerosol allows for deposition of nicotine with the respiratory system, including deep lung deposition. The vaporizing device may comprise a vaporization unit, a battery, and an integrated circuit coupled to the battery, wherein the integrated circuit is configured to control the battery for rapid initial vaporization without overheating, producing thermal degradation products, or draining battery energy. The battery may operate with pulse width modulation for at least a portion of the time the vaporizing device is being used.
Owner:NJOY LLC
Systems and methods for the delivery of corticosteroids
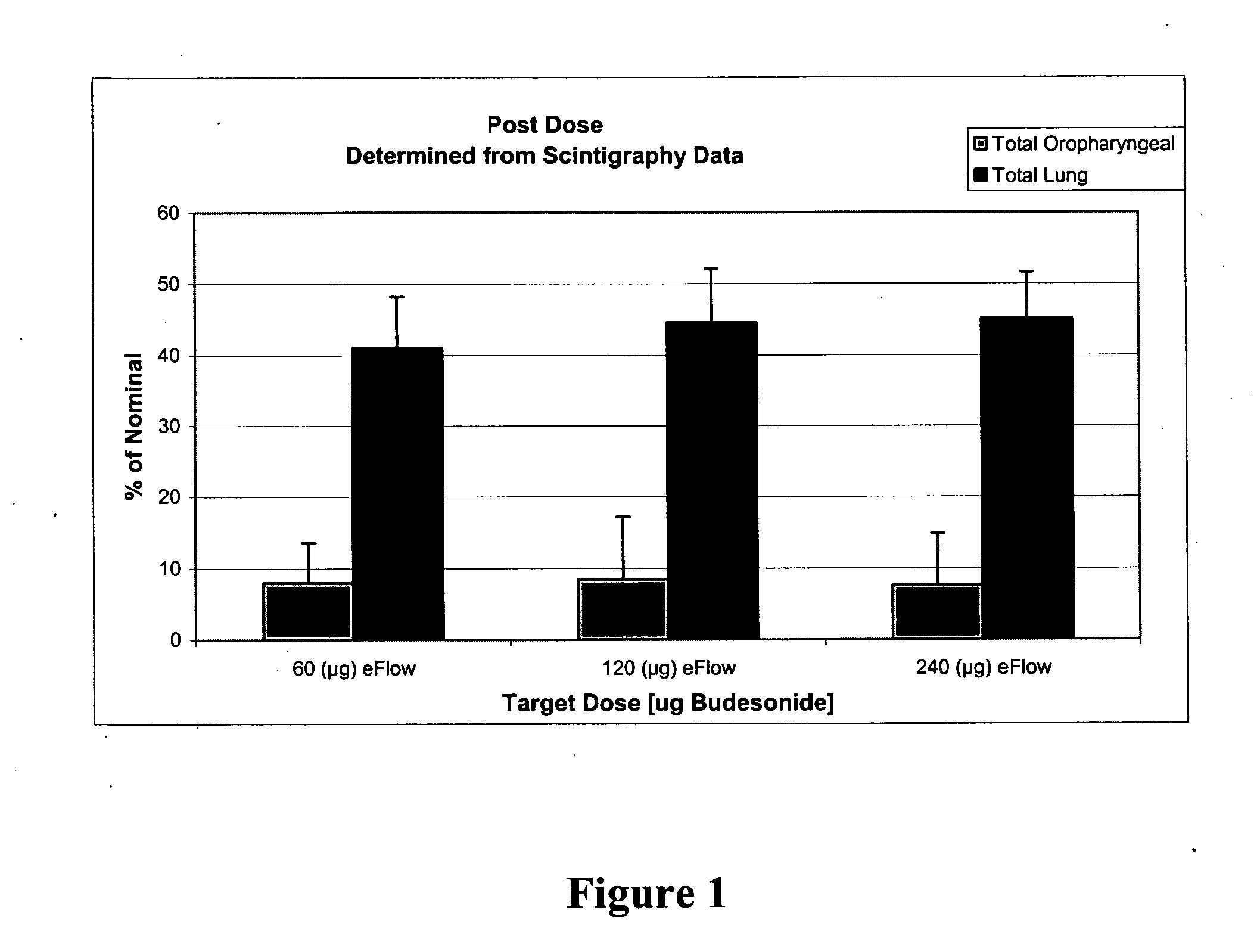
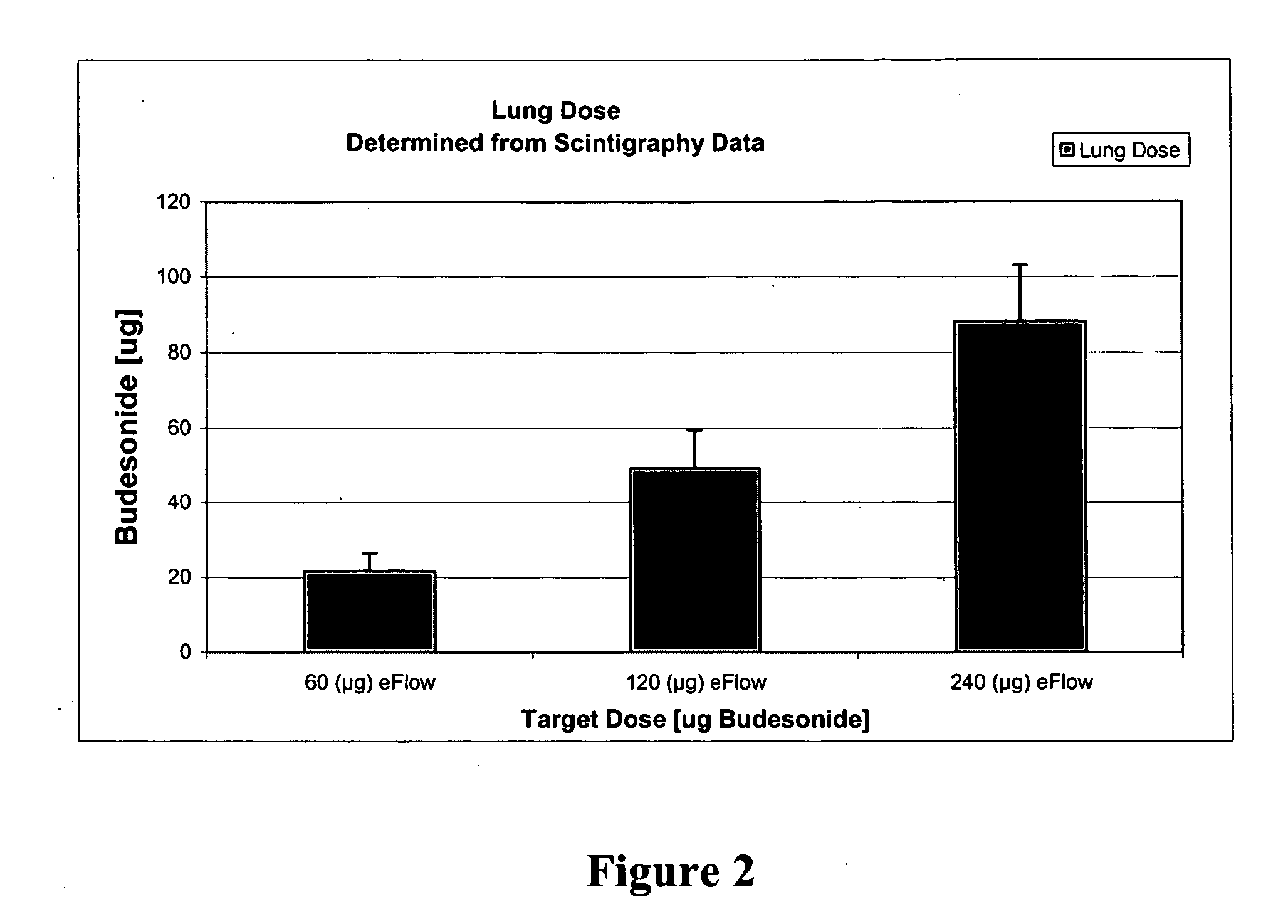
The present invention relates to methods and systems for the delivery of a corticosteroid comprising (1) an inhalable aqueous mixture comprising a corticosteroid and a solubility enhancer and (2) an inhalable nebulizer, wherein the delivery of the aqueous mixture comprising the corticosteroid by the nebulizer results in an enhanced pharmacokinetic profile of the corticosteroid as compared to conventional inhalable therapies and / or increase lung deposition.
Owner:TIKA LAEKEMEDEL AB
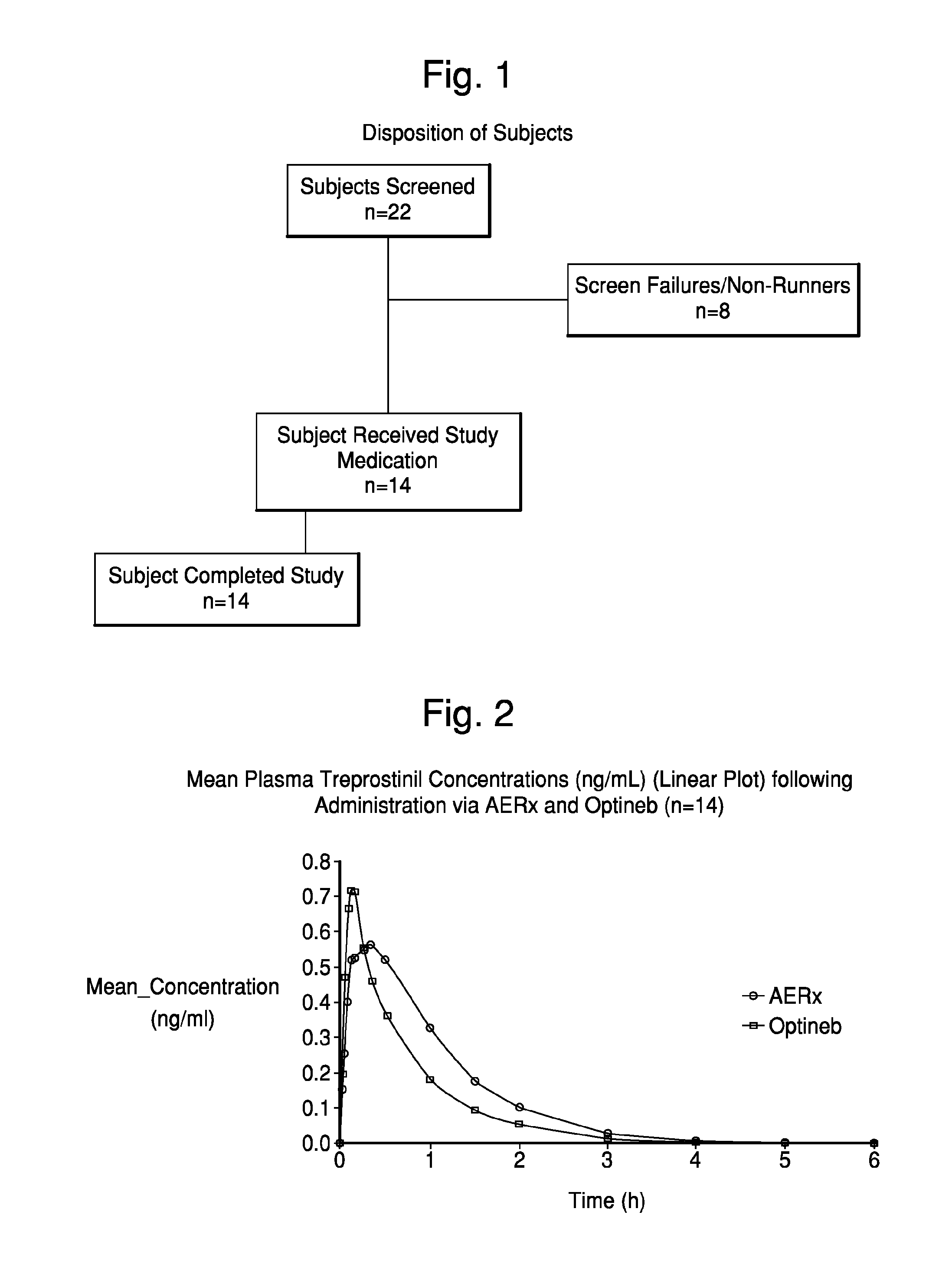
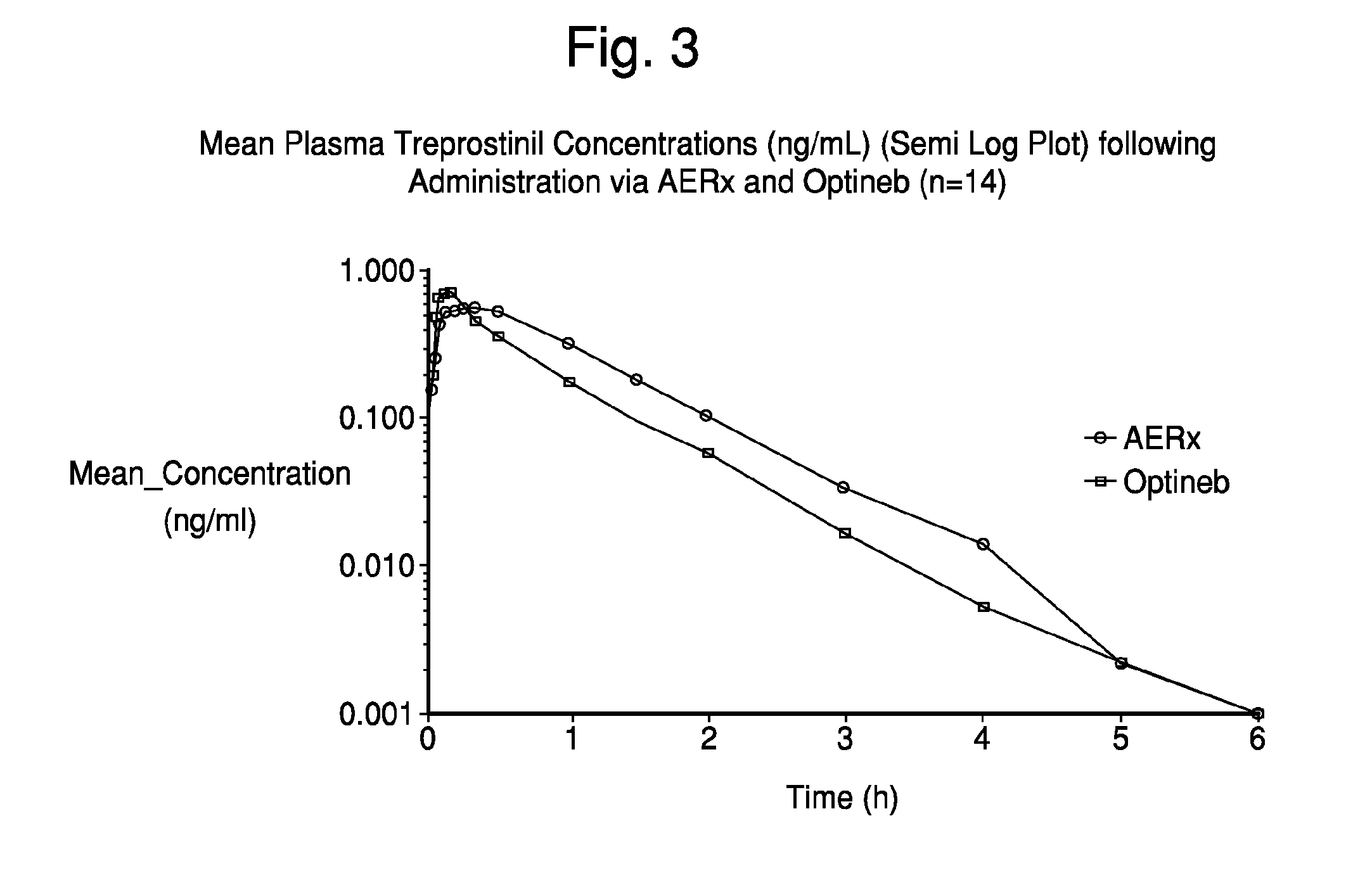
Deep lung pulmonary delivery of treprostinil
InactiveUS20120177693A1Slow onsetHigh selectivityBiocideDispersion deliveryTreprostinilIntensive care medicine
Administration of aerosolized Treprostinil formulations may provide a more homogeneous lung deposition of treprostinil, whereby making deep lung delivery possible.
Owner:ARADIGM
Methods and systems for the delivery of corticosteroids
The present invention relates to methods and systems for the delivery of a corticosteroid comprising (1) an inhalable aqueous mixture comprising a corticosteroid and a solubility enhancer and (2) an inhalable nebulizer, wherein the delivery of the aqueous mixture comprising the corticosteroid by the nebulizer results in an enhanced pharmacokinetic profile of the corticosteroid as compared to conventional inhalable therapies and / or increase lung deposition.
Owner:TIKA LAEKEMEDEL AB
Systems and methods for the delivery of corticosteroids having an increased lung deposition
The present invention relates to methods and systems for the delivery of a corticosteroid comprising (1) an inhalable aqueous mixture comprising a corticosteroid and a solubility enhancer and (2) an inhalable nebulizer, wherein the delivery of the aqueous mixture comprising the corticosteroid by the nebulizer results in an enhanced pharmacokinetic profile of the corticosteroid as compared to conventional inhalable therapies.
Owner:TIKA LAEKEMEDEL AB
Oral lung inhalation aerosol powder
ActiveCN101756942ALittle side effectsReduce dosagePharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicineBULK ACTIVE INGREDIENT
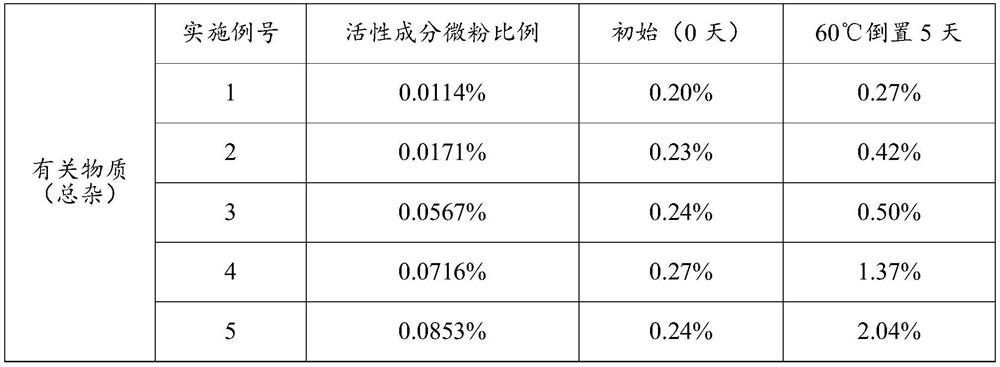
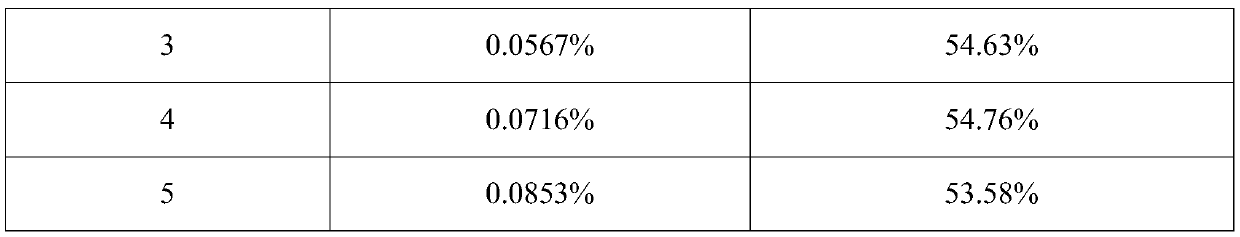
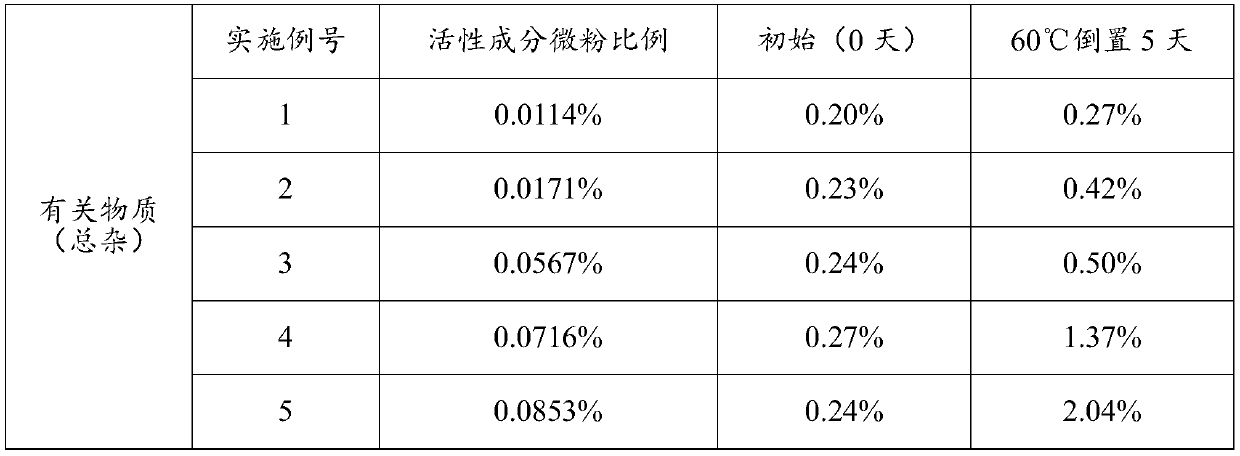
The invention discloses an oral lung inhalation aerosol powder, which can improve the lung deposition rate of active ingredients of the aerosol powder, avoid the deposition of carrier micropowder in the lung, and increase the stability of the composition. The oral lung inhalation aerosol powder consists of the active ingredients and the carrier micropowder. The oral lung inhalation aerosol powder is characterized in that: the mean grain size of the micropowder of the active ingredients is between 0.5 and 10 mu m, and the mean grain size of the carrier micropowder is between 20 and 45 mu m.
Owner:TIANJIN JINYAO GRP
Methods and systems for the delivery of corticosteroids having an enhanced pharmacokinetic profile
InactiveUS20070160542A1Improved pharmacokinetic profileImproved profileOrganic active ingredientsBiocideSolubilityNebulizer
The present invention relates to methods and systems for the delivery of a corticosteroid comprising (1) an inhalable aqueous mixture comprising a corticosteroid and a solubility enhancer and (2) an inhalable nebulizer, wherein the delivery of the aqueous mixture comprising the corticosteroid by the nebulizer results in an enhanced pharmacokinetic profile of the corticosteroid as compared to conventional inhalable therapies and / or increased lung deposition.
Owner:TIKA LAEKEMEDEL AB
Systems and methods for the delivery of corticosteroids
The present invention relates to methods and systems for the delivery of a corticosteroid comprising (1) an inhalable aqueous mixture comprising a corticosteroid and a solubility enhancer and (2) an inhalable nebulizer, wherein the delivery of the aqueous mixture comprising the corticosteroid by the nebulizer results in an enhanced pharmacokinetic profile of the corticosteroid as compared to conventional inhalable therapies and / or increase lung deposition.
Owner:TIKA LAEKEMEDEL AB
Systems and methods for the delivery of corticosteroids having an enhanced pharmacokinetic profile
InactiveUS20070178049A1Improve bioavailabilityLocal bioavailability of the budesonideOrganic active ingredientsBiocideSolubilityNebulizer
The present invention relates to methods and systems for the delivery of a corticosteroid comprising (1) an inhalable aqueous mixture comprising a corticosteroid and a solubility enhancer and (2) an inhalable nebulizer, wherein the delivery of the aqueous mixture comprising the corticosteroid by the nebulizer results in an enhanced pharmacokinetic profile of the corticosteroid as compared to conventional inhalable therapies, and / or increased lung deposition.
Owner:TIKA LAEKEMEDEL AB
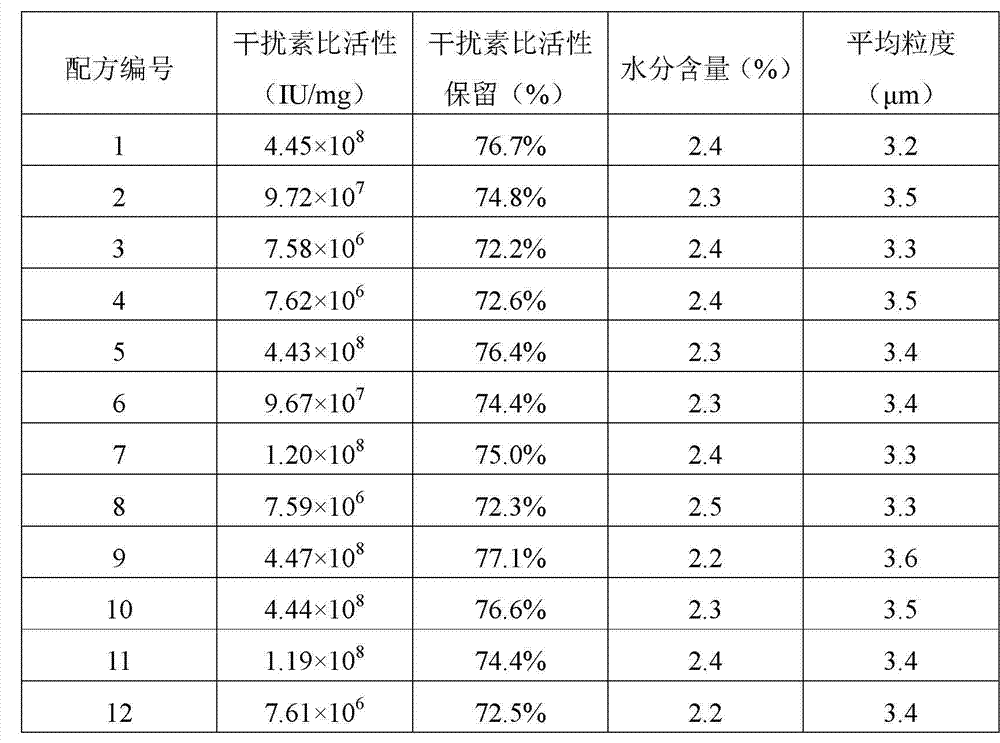
Dry powder inhalant of interferon alpha
The invention relates to a dry powder inhalant of an interferon alpha, belonging to the field for agents of protein drug, wherein the inhalant comprises 1-100 parts of interferon alpha, 3,000-4,000 parts of leucine, 0-4,000 parts of threonine, 12,000-16,000 parts of mannitol, 400 to 10,000 parts of buffer salt system with the pH controlled within the range of 6.0 to 8.0 by weight; and the inhalant does not comprise large-granularity carriers. The dry powder inhalant of the interferon alpha without the large-granularity carriers can achieve basically the same lung deposition effect with the powder inhalant of the interferon alpha with the large-granularity carriers in the in-vitro simulated deposition test.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
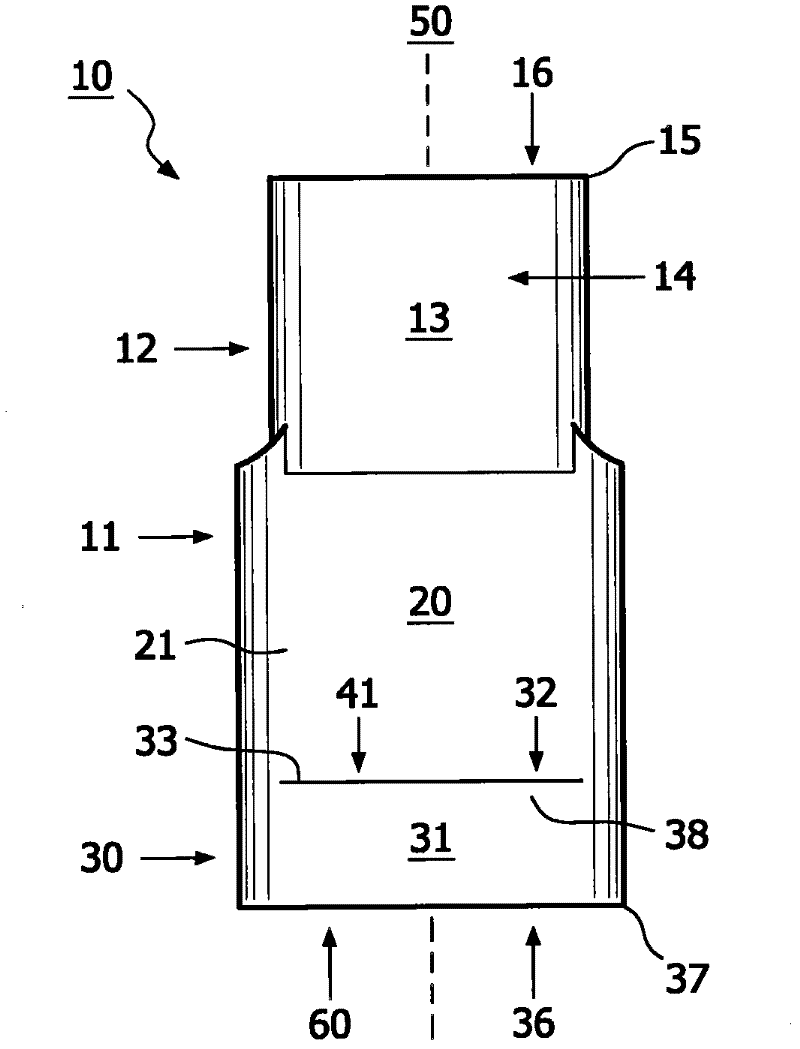
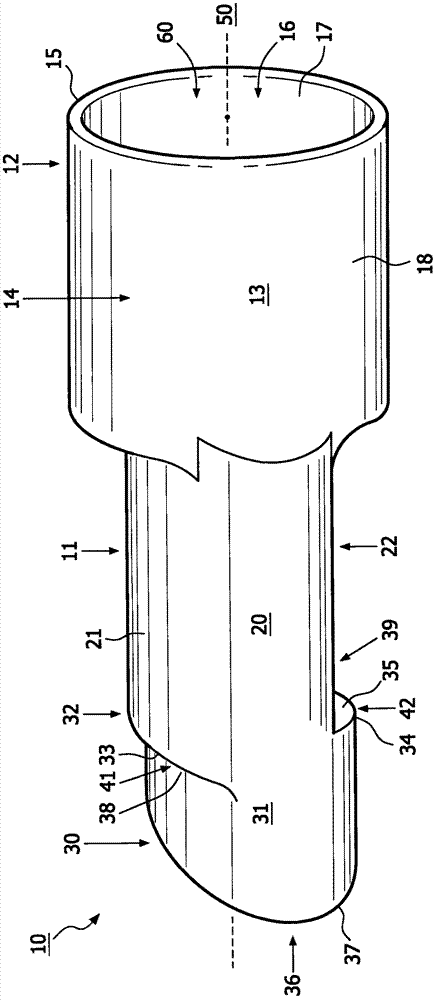
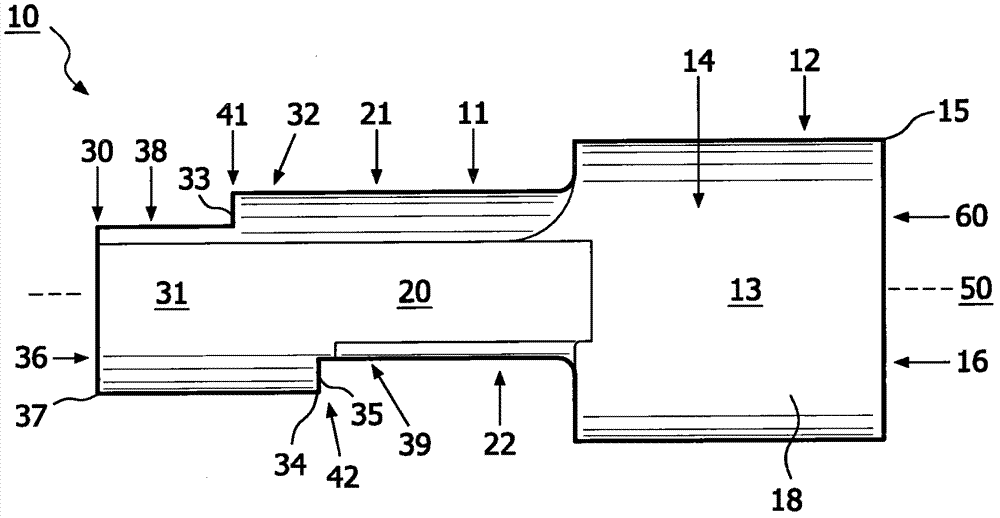
Method for aerosol drug delivery and device including stepped mouthpiece
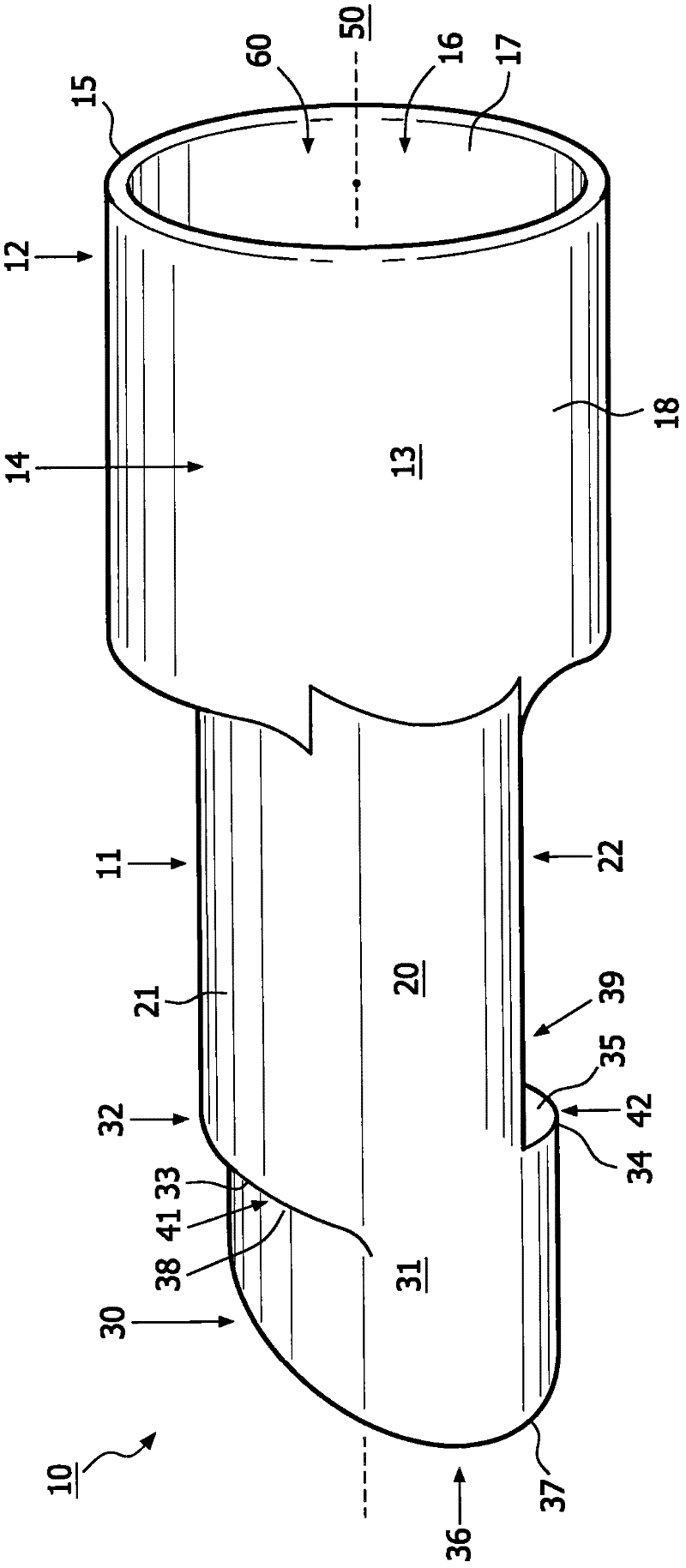
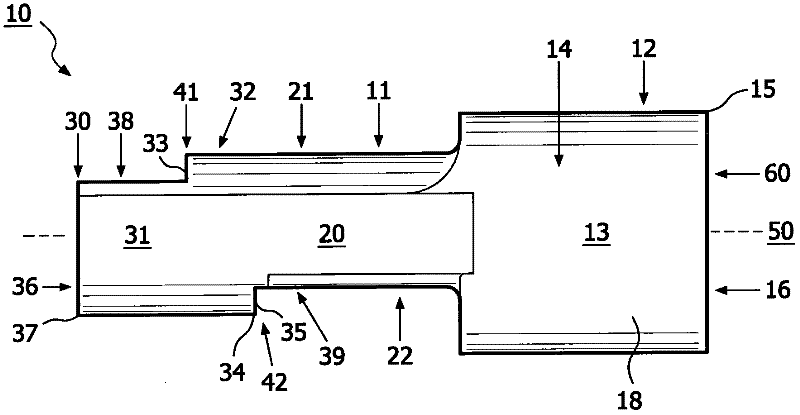
The present invention relates to an apparatus (10) to aid in administering inhaled pharmaceutical aerosol to a patient. The apparatus (10) is used in conjunction with an aerosol delivery device. The apparatus comprises steps (32, 34) on the top and bottom of the apparatus (10), which when used aid the patient causes mandibular advancement, and opening of the mouth, causing opening of patient's airway, resulting in improved aerosol lung deposition. The invention also relates to a method of using such apparatus in a combination with an aerosol delivery device or a system, and to the mouthpiece of said apparatus.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Asarone dry powder inhalant and method for preparing same
InactiveCN102614154ASimple processHigh rate of lung depositionPharmaceutical delivery mechanismEther/acetal active ingredientsAsaroneObstructive Pulmonary Diseases
The invention discloses an asarone dry powder inhalant and a method for preparing the same. The method for preparing the asarone dry powder inhalant includes steps: mixing asarone with auxiliary materials, and allowing the mixture to contact with high-speed air flow with crushing pressure being 0.4-0.7Mpa to be crushed to obtain micro powder, wherein the mass percentage content of the asarone ranges from 50% to 100%, and the mass percentage content of the auxiliary materials ranges from 0% to 50%; and mixing the micro powder with a medically acceptable carrier to obtain the asarone dry powder inhalant. The asarone dry powder inhalant is a novel agent and simple in preparation process, asarone medicine can be micronized, and lung deposition rate is increased. Further, the asarone dry powder inhalant has the advantages of quickness in effect, convenience in use and carrying, high stability and the like and is clinically widely applicable to treatment of asthma, chronic obstructive pulmonary disease and the like.
Owner:广州万泽医药科技有限公司
Preparation method and application of CO2 responsive micro-nano drug delivery system
ActiveCN112791068AEfficient depositionGood treatment effectOrganic active ingredientsPharmaceutical non-active ingredientsFreeze-dryingDimethyl acetal
The invention relates to a preparation method and application of a CO2 responsive micro-nano drug delivery system, which can effectively achieve the effects of improvement of lung deposition capability, reduction of systemic distribution of drugs, reduction of systemic toxic and side effects, responsive slow release in the lung, and improvement of drug utilization and curative effect. The preparation method comprises the following steps: preparing a polylactic acid-polylysine polymer; dissolving the polylactic acid-polylysine polymer in dimethyl sulfoxide, then adding N, N-dimethylacetamide dimethyl acetal, carrying out a nitrogen protection reaction, dialysis and freeze-drying, dissolving the obtained solution and budesonide in dimethyl sulfoxide, adding ultrapure water, and carrying out dialysis and freeze-drying to obtain PLA-mPLL / BUD nanoparticles; preparing yeast microcapsules, dissolving the PLA-mPLL / BUD nanoparticles in ultrapure water, adding the yeast microcapsules, carrying out vortex uniform mixing, carrying out room temperature incubation, centrifuging, and carrying out freeze drying to obtain the CO2 responsive micro-nano drug delivery system YGM / PLA-mPLL / BUD. The preparation method is simple, raw materials are rich, production and preparation are easy, and the system is a great innovation in medicines for treating asthma, pulmonary fibrosis, chronic pulmonary obstruction pulmonary disease, pneumonia and lung injury.
Owner:ZHENGZHOU UNIV
Inhalation training instrument for pulmonary inhalation administration
PendingCN112206386AGood treatment effectIncrease deposition rateInhalatorsPulmonary inhalationFlow transducer
The invention belongs to the technical field of medical instruments and provides an inhalation training instrument for pulmonary inhalation administration. The inhalation training instrument comprisesa tank, an inhalation cylinder, a resistance regulator, a flow sensor, a signal converter and a data analysis part. The inhalation cylinder can rotate around the axis of the tank. Along with rotationof the inhalation cylinder, fan-shaped regulating holes in the resistance regulator coincide with different vent holes, so that resistance borne by airflow is regulated, the flow sensor measures actual flow, the actual flow is converted by the signal converter and then transmitted to the data analysis part, the data analysis part calculates inhalation acceleration, inhalation peak flow velocity and inhalation volume according to flow data, and the data are displayed through a displayer of the data analysis part. According to the inhalation training instrument for pulmonary inhalation administration, the data results such as the inhalation acceleration, the inhalation peak flow velocity and the inhalation volume are visualized, a patient is helped to train the inhalation mode, the pulmonary deposition rate of medicine and the uniformity of delivery dosage are improved, and the patient can obtain a better treatment effect in inhalation medicine treatment.
Owner:张玮
Powder aerosol capable of resisting to idiopathic pulmonary fibrosis and preparation method thereof
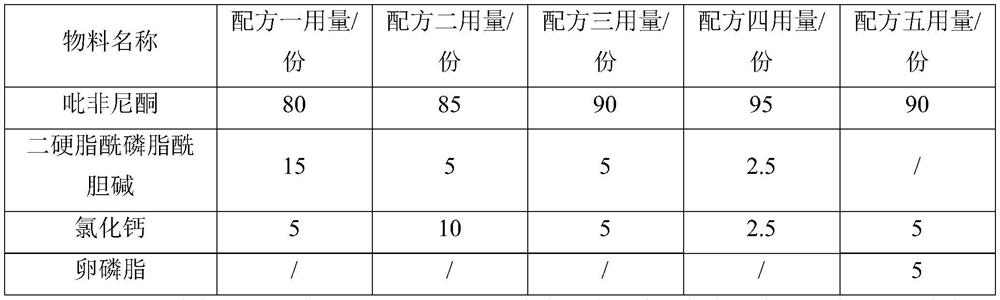
PendingCN113318097ASmall doseReduce adverse reactionsOrganic active ingredientsPowder deliveryActive agentDecreased surfactant
The invention relates to a powder aerosol capable of resisting to idiopathic pulmonary fibrosis and a preparation method thereof, and belongs to the field of medicinal preparations. The powder aerosol includes, by weight in parts, 80-95 parts of active pharmaceutical ingredients, 2.5-20 parts of an endogenous pulmonary surfactant, and 0-10 parts of calcium salt. The particle volume geometric diameter of the powder aerosol is 0.5-6 [mu]m. The powder aerosol has a less and narrow geometric diameter distribution, so that the powder aerosol can directly reach the lesion. A lung deposition rate FPF value is greater than 50%. An FPF value (3.4) is greater than 30%, which refers to a deep lung deposition pattern. The powder aerosol can be delivered to the depth of the lung to achieve a clinical effect of anti-pulmonary fibrosis; the dosage of active ingredients of drugs can be reduced; and therefore, adverse reactions caused by the increasing of the dosage of the active ingredients can be reduced to a greater extent.
Owner:ZHUHAI RESPROLY PHARM TECH CO LTD
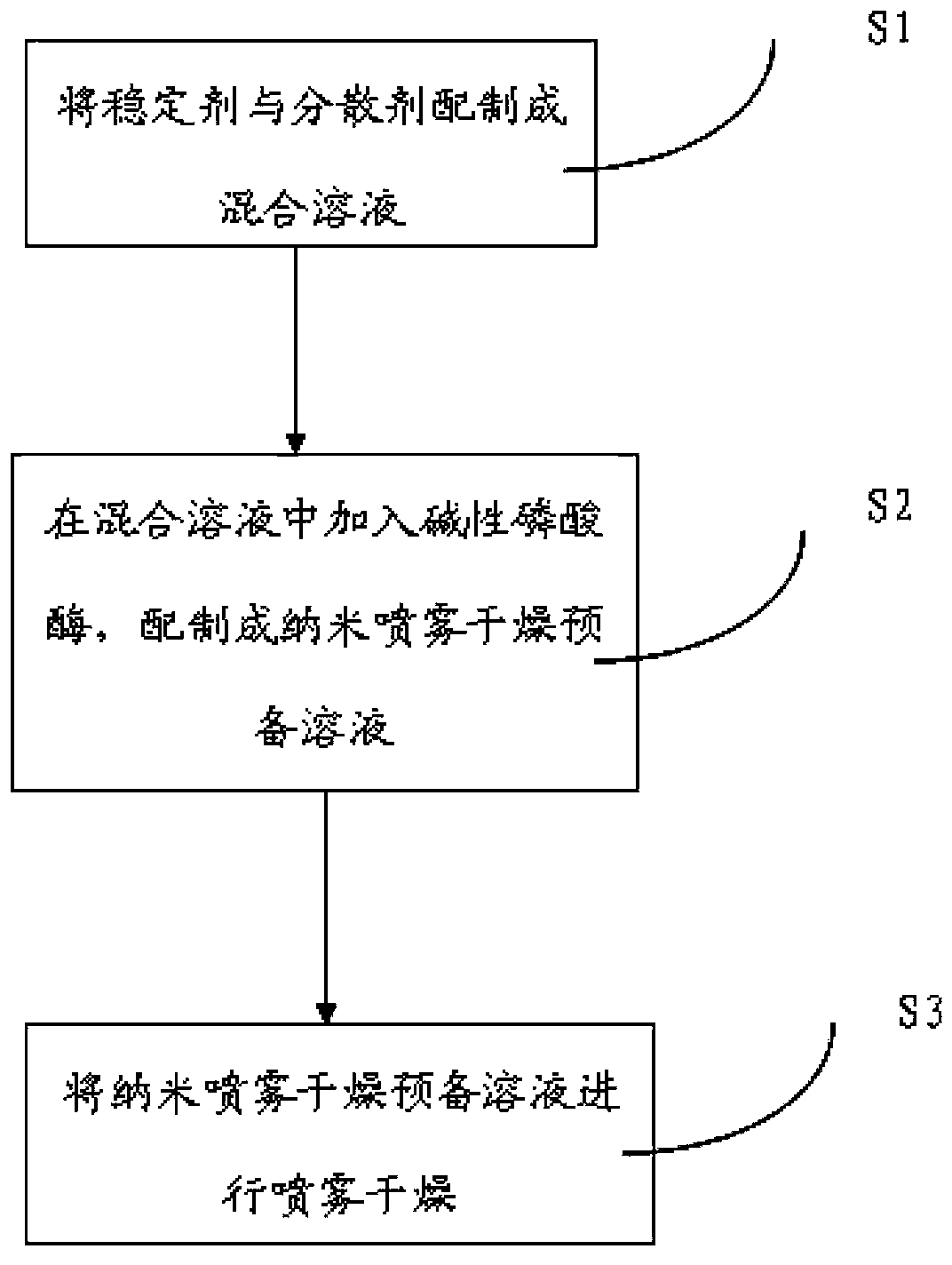
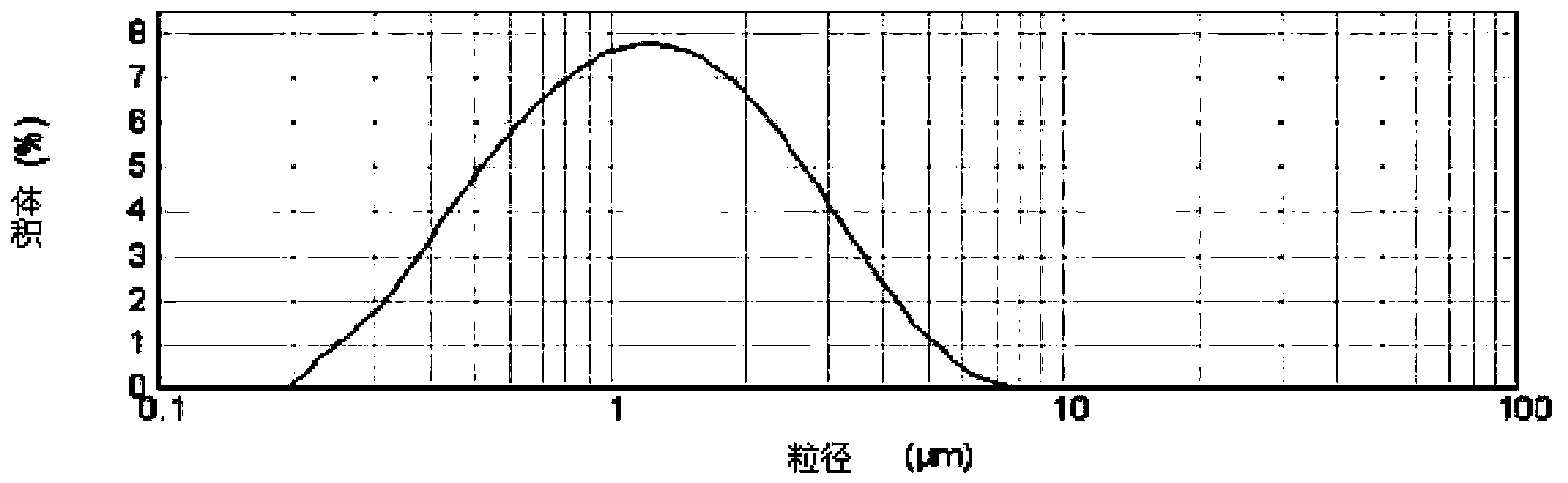
Alkaline phosphatase micro-/nano-particle and preparation method thereof
ActiveCN102743339BSmall particle sizeUniform particle sizePowder deliveryPeptide/protein ingredientsAdjuvantPatient compliance
The invention discloses an alkaline phosphatase micro- / nano-particle which comprises alkaline phosphatase as drug and adjuvants at weight ratio of 1:100-100:1. The adjuvants comprise stabilizer and dispersant at weight ratio of 1:100-100:1. The micro- / nano-particle has yield of 20-98%, average particle size of 0.1-10 micrometer, bio-active drug activity retention rate of 50-100%, and in-vitro lung deposition efficiency of 10-98%. The micro- / nano-particle with small and uniform particle size and good dispersivity can be delivered into lung in multiple lung inhalation preparation forms, can effectively maintain drug bioactivity, and has excellent lung inhalation efficiency, no gastrointestinal tract degradation effect and no liver first pass effect during application course, and good patient compliance due to non-invasive treatment manner.
Owner:常熟紫金知识产权服务有限公司
A kind of β2 receptor agonist inhalation aerosol and products containing the inhalation aerosol
ActiveCN110840864BEfficient depositionImprove stabilityPowder deliveryOrganic active ingredientsAlkanePolyethylene glycol
The invention provides a β2 receptor agonist inhalation aerosol and products containing the inhalation aerosol. The β2 receptor agonist inhalation aerosol of the present invention is composed of active ingredient formoterol or its pharmaceutically acceptable salt, pharmaceutical carrier, pharmaceutical solvent, polyethylene glycol, povidone and hydrofluoroalkane propellant Composition, by adding the appropriate auxiliary material povidone into the product prescription, and strictly limiting the content of each component in the prescription, and using a combination of fluorocarbon-coated metal cans and quantitative metal valves as the container system; the product stability and effectiveness are improved. Pulmonary deposition has obvious advantages and good market prospects.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD +1
Oral lung inhalation aerosol powder
ActiveCN101756942BLittle side effectsReduce dosagePharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicineBULK ACTIVE INGREDIENT
Owner:TIANJIN JINYAO GRP
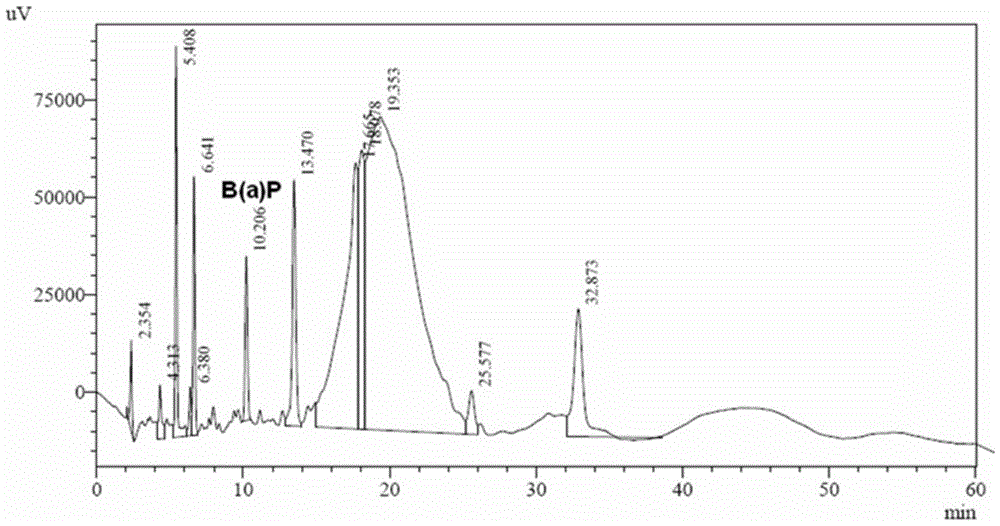
A method for determining the amount of benzopyrene deposited in the lungs of rats after smoking by using high performance liquid chromatography
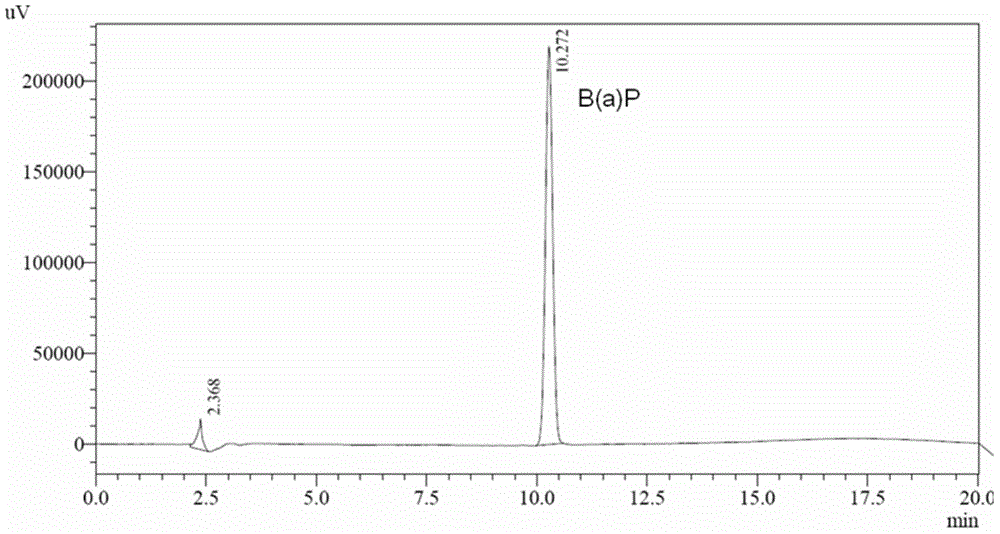
The invention relates to a method for determining deposition amount of benzopyrene in rat lung after smoking by virtue of high performance liquid chromatography. The method comprises five steps as follows: (1) inducing a rat to smoke; (2) preparing a to-be-detected sample solution; (3) preparing a standard working solution; (4) determining chromatographic conditions; and (5) drawing a standard curve and calculating a result. The method for determining deposition amount of benzopyrene in rat lung after smoking by virtue of high performance liquid chromatography disclosed by the invention has the following advantages: 1. through cigarette smoke generated from smoking by virtue of a smoking machine, deposition of benzopyrene in rat lung under different smoking modes of different cigarettes can be detected, and the operation is flexible; 2. compared with a conventional method adopting Soxhlet extraction, the experiment has the advantages that the sample processing time can be shortened greatly and the operation is convenient in a mode of cyclohexane extraction-dimethyl sulfoxide reextraction-cyclohexane extraction; and 3. the method disclosed by the invention has the advantages of high sensitivity and good repeatability and recovery rate.
Owner:CHINA TOBACCO ZHEJIANG IND
Method for preparing betamethasone dipropionate atomization inhalant
InactiveCN102973539AShort duration of actionHigh lung deposition rateOrganic active ingredientsSolution deliveryDispersion stabilitySide effect
The invention relates to the field of medical products, in particular to a method for preparing a betamethasone dipropionate atomization inhalant. The method comprises the following steps: dispersing betamethasone dipropionate into water to obtain suspension, homogenizing, sterilizing, and thus obtaining the betamethasone dipropionate atomization inhalant, wherein the homogenizing pressure is 200 to 2,000 bar. The preparation method is green and environment-friendly, simplifies the operation steps, can realize continuous operation, and is short in acting time, sterile, pollution-free and suitable for industrialized production. The prepared betamethasone dipropionate atomization inhalant has the advantages of high dispersion stability, high lung deposition rate, low medicament consumption, high bioavailability and low toxic or side effect.
Owner:SUZHOU UNIV
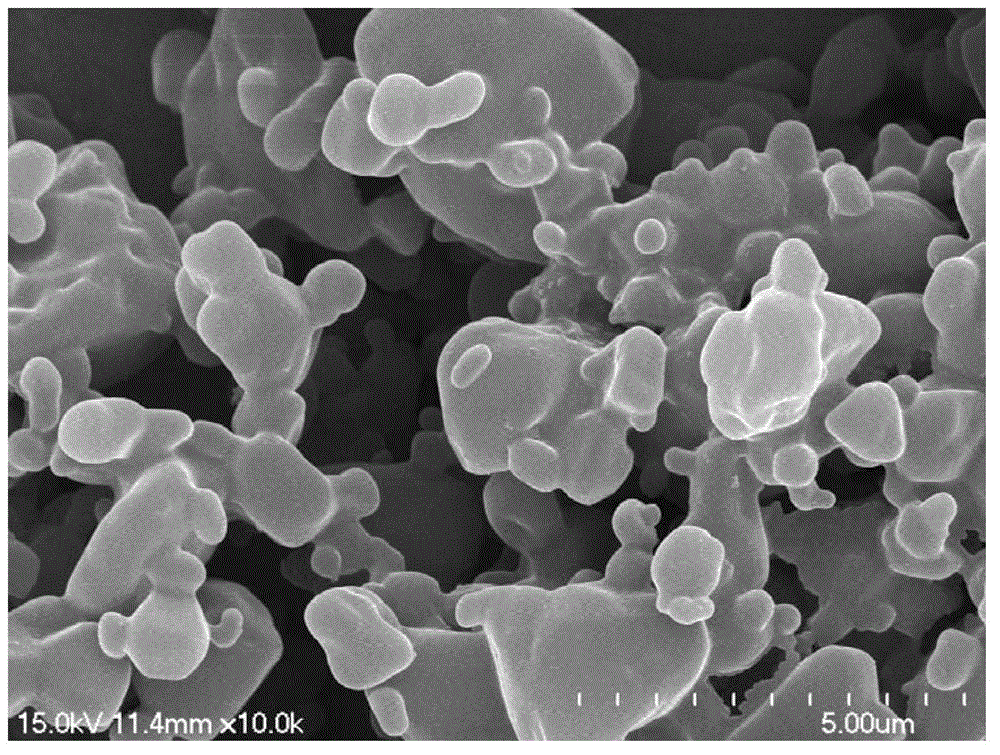
Preparation method for drug-carrier mode composite micro-powder
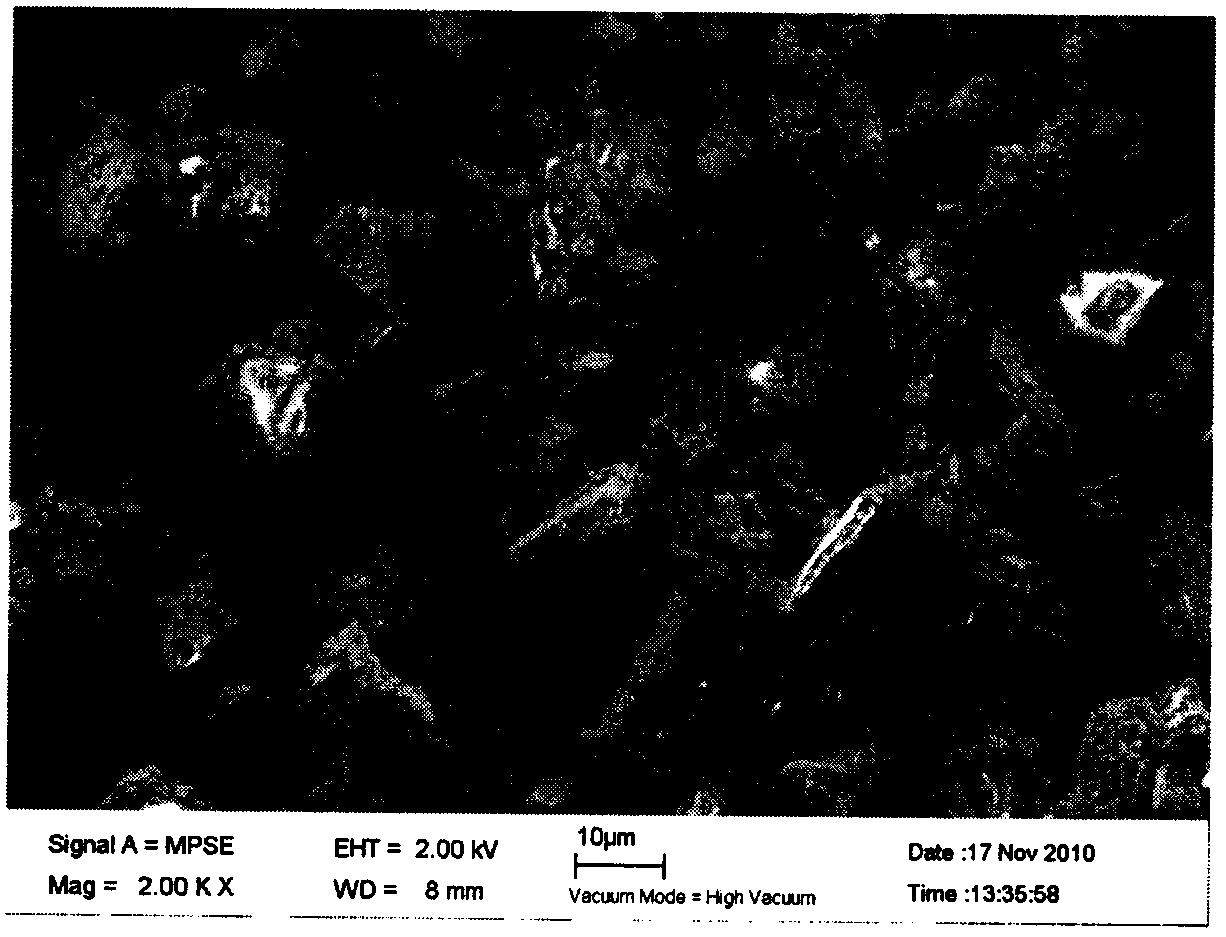
The invention discloses a preparation method for drug-carrier mode composite micro-powder. The composite micro-powder structure takes a fumed silica aggregate as a carrier; the fumed silica aggregate is located in the center of the composite micro-powder particles; drug particles are adsorbed on the surface of the carrier; the geometric particle size of the fumed silica aggregate is 1-30 microns; the geometric particle size of the drug particles is 10-1,000 nm; and the aerodynamic particle size of the composite micro-powder particles is less than 10 microns. The preparation method taking pentoxyverine citrate as a representative drug comprises the following steps: preparing a pentoxyverine citrate suspension with a high-pressure homogenization, reactive precipitation or anti-solvent precipitation method, wherein the geometric particle size of suspension particles is 10-1,000 nm; adding the fumed silica aggregate into the suspension, and mixing uniformly, wherein the mass percentage of the fumed silica aggregate to the pentoxyverine citrate is 15-60% to 40-85%; and spray-drying a mixed suspension to obtain the composite micro-powder. The preparation method is simple in process, low in cost and short in cycle. Compared with carrier-free mode micro-powder, the composite micro-powder has more excellent aerodynamic performance, lung deposition performance, flowability and dispersity.
Owner:中国人民解放军63975部队
a co 2 Preparation method and application of responsive micro-nano drug delivery system
ActiveCN112791068BEfficient depositionGood treatment effectOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical SubstancesBiology
Owner:ZHENGZHOU UNIV
Compositions, devices, and methods for nicotine aerosol delivery
The present disclosure generally relates to compositions, and related devices and methods, useful in vaporizing devices such as electronic cigarettes. The composition may comprise nicotine, at least one solvent, and at least one ion pairing agent, and may be vaporized to form a condensation aerosol, wherein inhalation of the aerosol allows for deposition of nicotine with the respiratory system, including deep lung deposition. The vaporizing device may comprise a vaporization unit, a battery, and an integrated circuit coupled to the battery, wherein the integrated circuit is configured to control the battery for rapid initial vaporization without overheating, producing thermal degradation products, or draining battery energy. The battery may operate with pulse width modulation for at least a portion of the time the vaporizing device is being used.
Owner:NJOY LLC
Deep lung pulmonary delivery of treprostinil
Administration of aerosolized Treprostinil formulations may provide a more homogeneous lung deposition of treprostinil, whereby making deep lung delivery possible.
Owner:ARADIGM
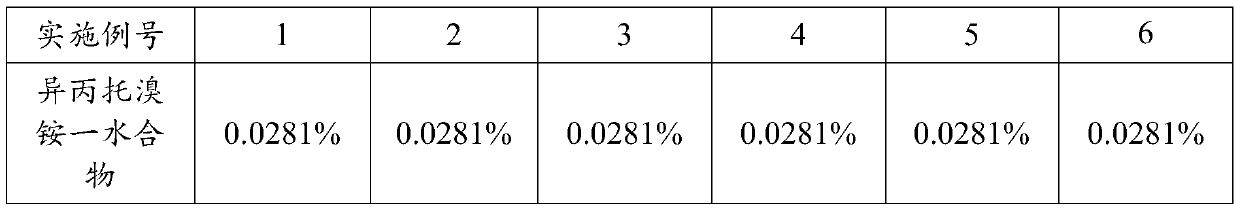
Inhalation aerosol containing anticholinergic drug, preparation process and application method of inhalation aerosol
ActiveCN111297835AGood curative effectShorten speedDispersion deliveryAerosol deliveryAlkaneUse medication
The invention provides an inhalation aerosol containing an anticholinergic drug, a preparation process and an application method of the inhalation aerosol. The inhalation aerosol consists of an activeingredient ipratropium bromide, or a monohydrate or a pharmaceutically acceptable salt thereof, a hydrofluoroalkane propellant, a latent solvent absolute ethyl alcohol and a pH regulator. The inhalation aerosol provided by the invention can achieve an effective lung deposition rate meeting requirements, and meanwhile, the drug stability is remarkably improved; meanwhile, the inhalation aerosol can be used in combined utilization with a mist storage device, the medication compliance of old people and children is improved, the curative effect of medicine used by patients is improved, and side effects are reduced.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Beta2 receptor agonist inhalation aerosol and product containing the inhalation aerosol
ActiveCN110840864AEfficient depositionImprove stabilityOrganic active ingredientsPowder deliveryAlkanePolyethylene glycol
The invention provides a beta2 receptor agonist inhalation aerosol and a product containing the inhalation aerosol. The beta2 receptor agonist inhalation aerosol consists of an active ingredient formoterol or a pharmaceutically-acceptable salt, a medicinal carrier, a medicinal solvent, polyethylene glycol, povidone and a hydrofluoroalkane propellant, and is prepared by adding a suitable auxiliarymaterial povidone into the product prescription, strictly limiting the content of each component in the prescription, and simultaneously combining a fluorocarbon-coated metal tank and a quantitative metal valve as a container system. The product stability and effective lung deposition are improved, and the product has obvious advantages and a good market prospect.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD +1
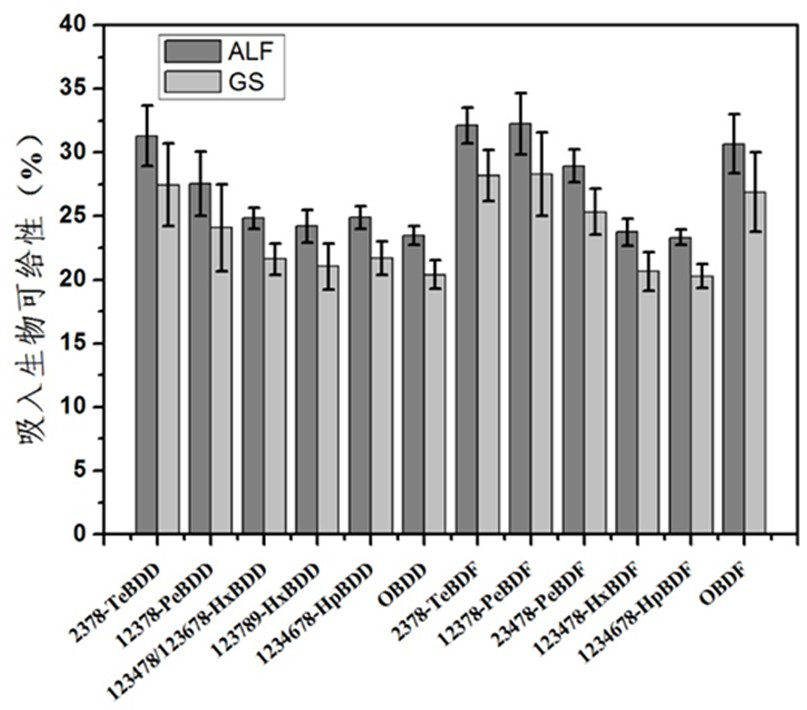
In-vitro simulation analysis system and method for PBDD/Fs in atmospheric particulates
ActiveCN113984594AInhalation bioavailability is conveniently obtainedInhalation bioavailabilityMaterial analysisTenaxSorbent
The invention provides an in-vitro simulation analysis system and method for PBDD / Fs in atmospheric particulates. The method comprises the following steps: (a) collecting an atmospheric particulate sample by using an atmospheric particulate sampler and a quartz fiber membrane, and analyzing the content of PBDD / Fs in the atmospheric particulate sample; (b) cutting the quartz fiber membrane into pieces, respectively putting the quartz fiber membrane pieces into a semi-permeable membrane bag, adding a Tenax adsorbent into a centrifugal tube, respectively adding an ALF simulation lung fluid and a GS simulation lung fluid into reagent bottles, and carrying out an in-vitro simulation experiment; (c) detecting the content of PBDD / Fs in the Tenax adsorbent and the simulation lung fluid; and (d) considering the lung deposition efficiency of atmospheric particulates, and evaluating the inhalation bioaccessibility of the carrier PBDD / Fs. According to the invention, the inhalation bioaccessibility of PBDD / Fs can be conveniently obtained, and the method has important significance for accurately evaluating the respiratory exposure risk of people with the PBDD / Fs in the particulate matters.
Owner:HEBEI UNIVERSITY
A preparation method of drug-additive mode composite micropowder
Owner:中国人民解放军63975部队
Method for aerosol drug delivery and apparatus comprising stepped mouthpiece
The invention of the present application relates to an apparatus to aid in administering inhaled pharmaceutical aerosol to a patient. The apparatus is used in conjunction with an aerosol delivery device. The apparatus comprises steps on the top and bottom of the apparatus, which when used aid the patient causes mandibular advancement, and opening of the mouth, causing opening of patient's airway, resulting in improved aerosol lung deposition. The invention also relates to a method of using such apparatus in a combination with an aerosol delivery device or a system, and to the mouthpiece of said apparatus.
Owner:KONINK PHILIPS ELECTRONICS NV
Preparing method of medicine-additive mode composite micro powder
The invention discloses a preparing method of medicine-additive mode composite micro powder. The composite micro powder is structurally characterized in that medicine particles with the geometric particle size of 1-10 microns are located in the centers, additive modified gas-phase white carbon black with the geometric particle size of 10-65 nm are adsorbed to the surfaces of the medicine particles, and the aerodynamics particle size of particles of the composite micro powder is smaller than 10 microns. The preparing method with pentoxyverine as representative medicine includes the following steps that a modifying agent for the modified gas-phase white carbon black is prepared from one or more of hexamethyldisilazane, cyclo-silazane and octyltriethoxysilane, and the geometric particle size of the modified gas-phase white carbon black is 10-65 nm; pentoxyverine turbid liquid is prepared, wherein the geometric particle size of turbid particles is 1-10 microns; the modified gas-phase white carbon black is added into the turbid liquid, wherein the mass percent of the additive to the medicine is 10-70%:30-90%; the mixed turbid liquid is subjected to spray drying, and the composite micro powder is obtained. The preparing method is simple in technology, low in cost and short in period. Compared with carrier-free-mode micro powder, the composite micro powder has the more excellent aerodynamics performance, the more excellent lung deposition performance, the more excellent fluidity and the more excellent dispersion degree.
Owner:中国人民解放军63975部队
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com