Asarone dry powder inhalant and method for preparing same
A technology of brain-dry powder and inhalant, which is applied in the field of medicine, can solve the problems of inability to be directly freeze-dried, the safety impact of inhalation preparations, and the inability to evaporate solvents, etc., and achieve the effects of fast onset, easy portability, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of Asarum dry powder inhalation comprises the steps:
[0032] (1) After asarone is mixed with auxiliary materials, it is contacted and crushed with a high-speed airflow with a crushing pressure of 0.4 to 0.7Mpa to obtain micropowder; the auxiliary materials are one or more of magnesium stearate, lactose, and mannitol; The mass percentage of the asarone is 50% to 100%, and the mass percentage of the auxiliary material is 0% to 50%;
[0033] (2) Mix the micropowder obtained in step (1) with a medically acceptable carrier, collect it in capsules or aluminum-plastic blisters, or directly pack it into an inhalation device to obtain the product.
[0034] Asarone in the present invention is a natural extract, an artificial compound or a mixture of the two.
Embodiment 1
[0035] The preparation method of embodiment 1 Asarum dry powder inhalation
[0036] Including the following steps:
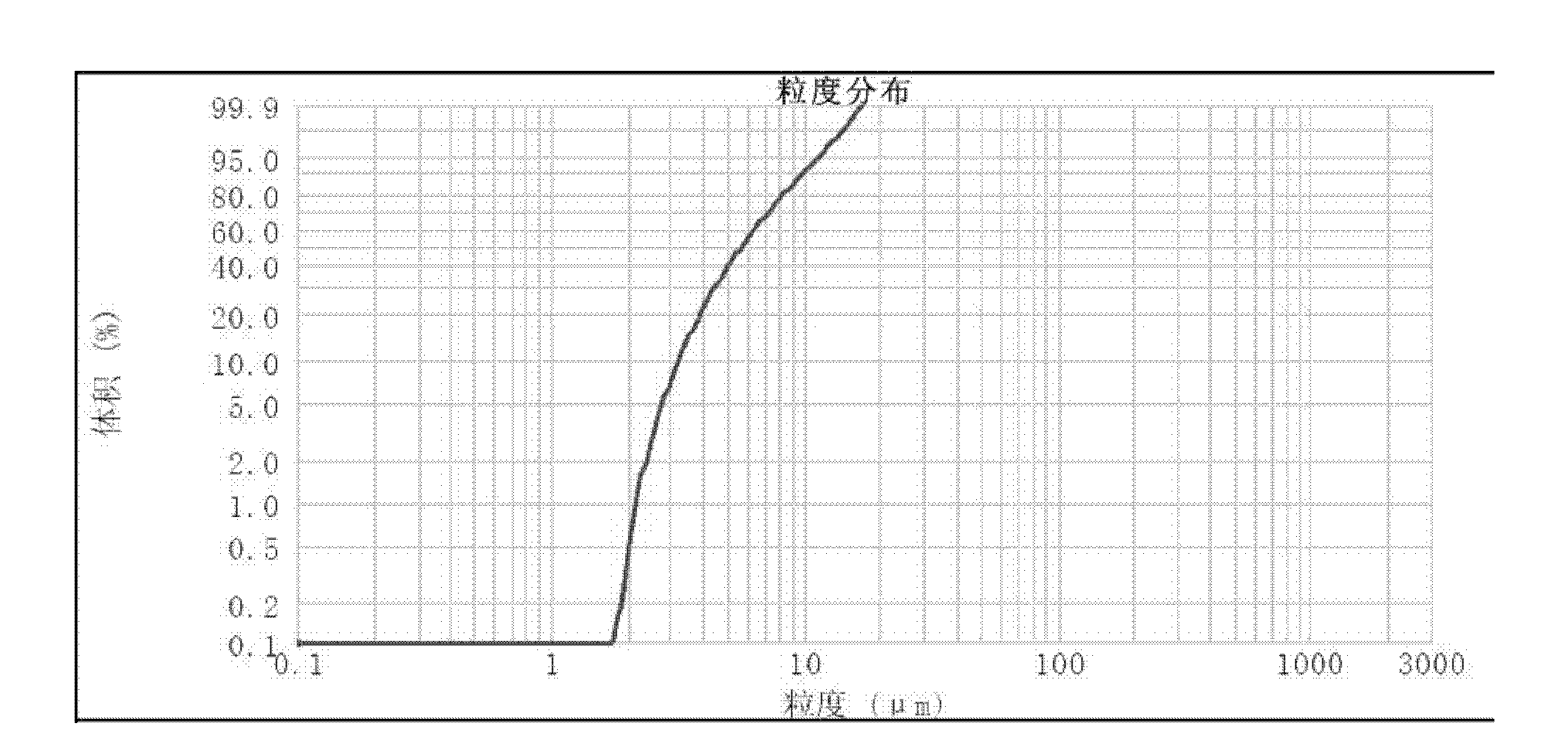
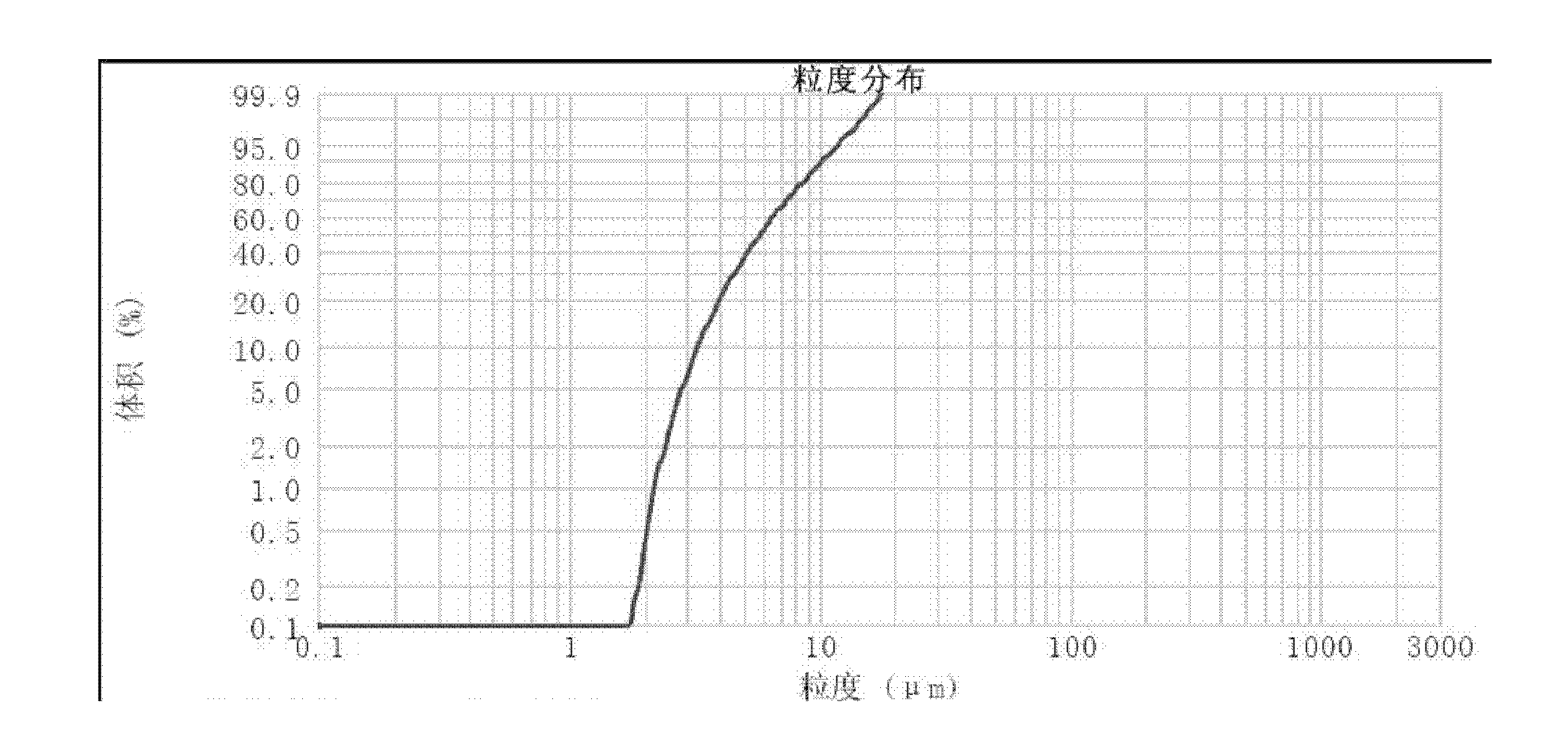
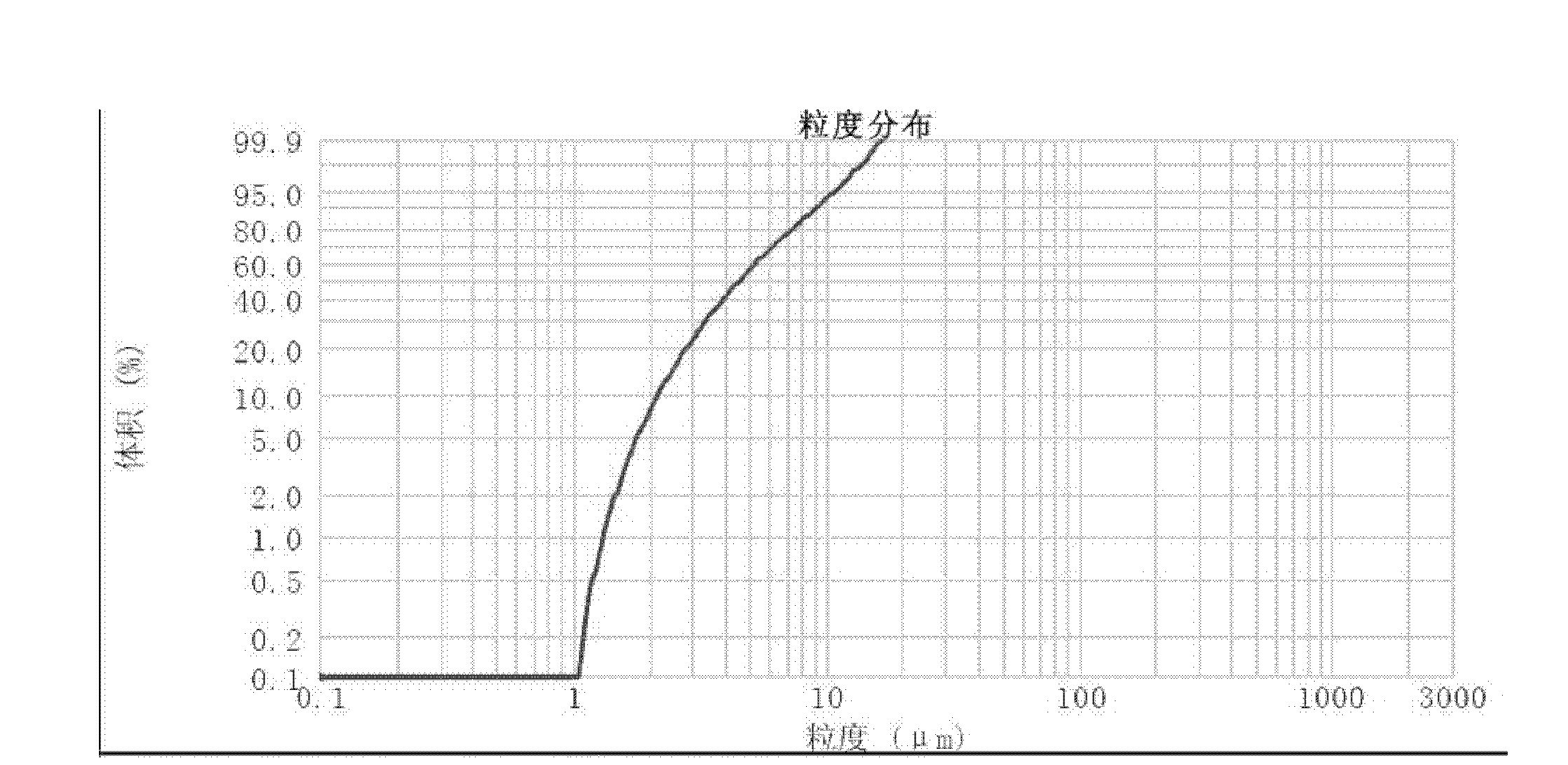
[0037] (1) after asarone and lactose (adjuvant) are mixed uniformly, be that the high-speed air flow of crushing pressure is 0.7Mpa in jet mill and pulverize, obtain micropowder; The mass percentage composition of described asarone is 50%, The mass percentage of the lactose is 50%; the micropowder yield is 21.79%; the micropowder content below 5 μm is 37.90%;
[0038] (2) Mix the micropowder obtained in step (1) with lactose (carrier) evenly, and pack them in 3# HPMC plant capsules to obtain dry asarum powder inhalation.
Embodiment 2
[0039] The preparation method of embodiment 2 Asarum dry powder inhalation
[0040] Including the following steps:
[0041] (1) After asarone and magnesium stearate (adjuvant) are mixed homogeneously, be the high-speed air flow contact pulverization of 0.4Mpa with crushing pressure in jet mill, obtain micropowder; The mass percentage composition of described asarone is: 99.5%, the mass percentage of the magnesium stearate is 0.5%; the micropowder yield is 12.42%; the micropowder content below 5 μm is 35.97%;
[0042] (2) Mix the micropowder obtained in step (1) with lactose (carrier) evenly, and pack them in 3# HPMC plant capsules to obtain dry asarum powder inhalation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com