Deep lung pulmonary delivery of treprostinil
A technology of treprostinil and lung deposition, applied in the field of lung delivery, can solve the problem of not providing sustained drug release, and achieve the effect of increasing efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 9. Research plan
[0074] 9.1 General study design and plan
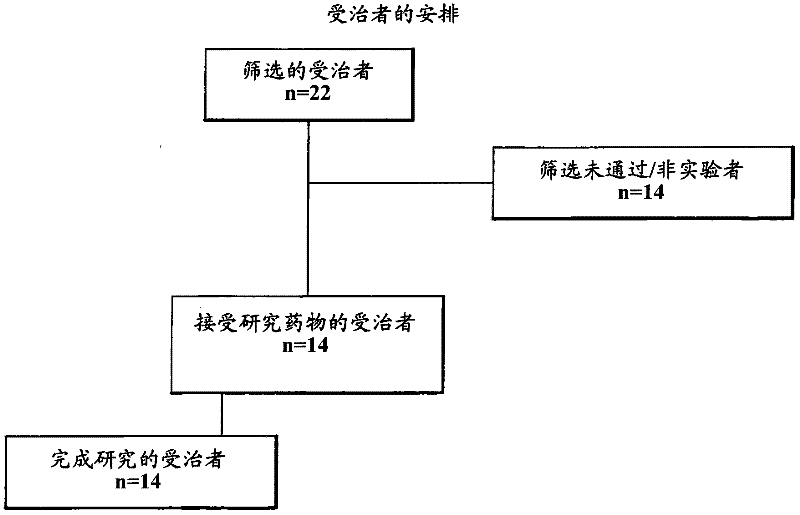
[0075] This was a single-centre, open-label study using a randomized two-way crossover design. Sixteen healthy adult male subjects participated and received study treatment. After providing written informed consent, each study candidate underwent a pre-study assessment and screening to determine eligibility for participation.
[0076] Subjects were instructed and trained in the proper use of the Nebu-Tec Optineb Nebulizer and the use of the drug-free AERx Essence System.
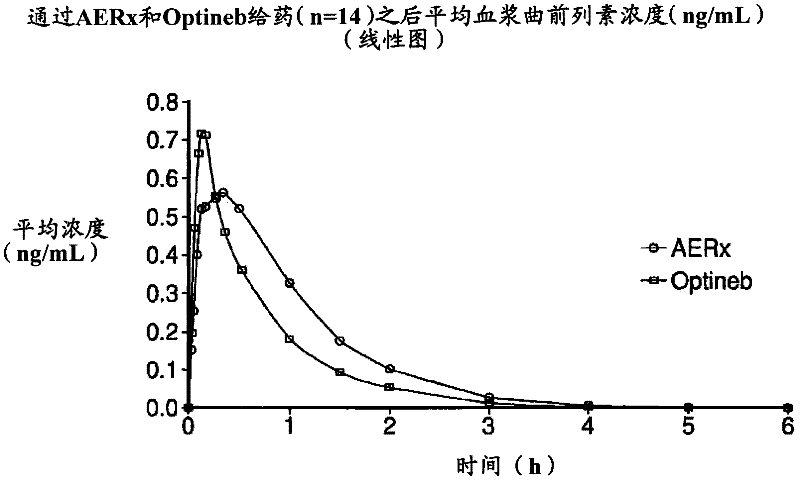
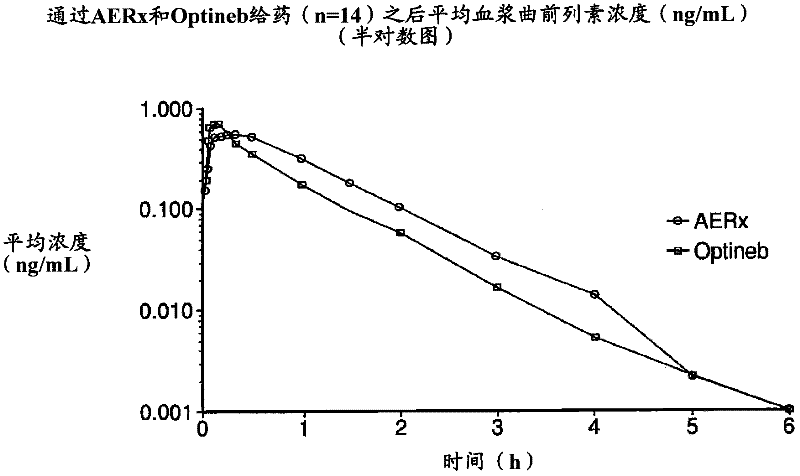
[0077] On each of the two dosing days, eligible subjects administered either the AERx Essence System or the Nebu-Tec Optineb Nebulizer 99m Administration of Tc-labeled treprostinil sodium. Following the initial study dose, subjects underwent a washout period of approximately 48 hours before completing the second (crossover) study dose.
[0078] Following each study dose, subjects underwent gamma scintigraphy and multiple venous blood s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com