A preparation method of drug-additive mode composite micropowder
A technology of additives and drugs, applied in the field of drug-additive mode composite micropowder preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] figure 1 It is the scanning electron microscope microscopic morphology of fumed silica. The geometric particle size of fumed silica particles is 5-20nm, the shape is irregular, and there is obvious agglomeration between particles.
Embodiment 2
[0030] figure 2 It is the scanning electron microscope microscopic morphology of fumed silica modified by hexamethylcyclotrisilazane. Hexamethylcyclotrisilazane modified fumed silica particles have a geometric particle size of 10-20nm, a regular spherical shape, and no obvious agglomeration.
[0031] Modifier hexamethylcyclotrisilazane modified fumed silica process: add 10g of fumed silica to the reactor, stir continuously at room temperature, add 1g of modifier dropwise, and the reaction by-product ammonia is condensed by reflux After the modifier is added dropwise, continue to stir for 1 hour; after the modification, nitrogen gas is introduced to completely remove the residual ammonia; centrifuge and wash with ethanol for 2-3 times, and dry to constant weight.
[0032] When the same modification process is adopted, the hexamethyldisilazane-modified fumed silica particles have a geometric particle size of 10-30 nm, a regular spherical shape, and no obvious agglomeration. T...
Embodiment 3
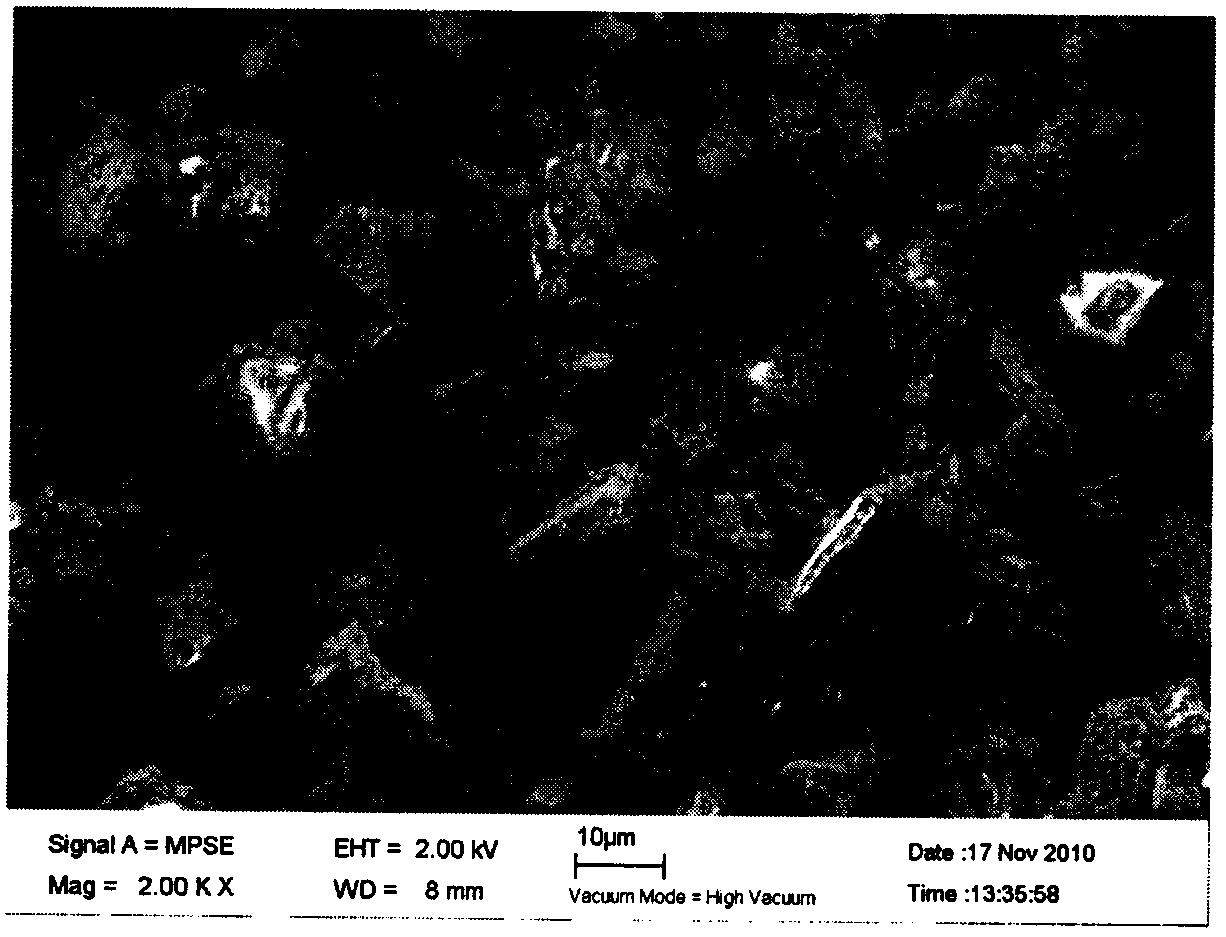
[0034] Pentoxyverine citrate is a widely used non-addictive antitussive. In addition to its direct inhibitory effect on the respiratory center, it also has a weak atropine effect, which can slightly inhibit the endobronchial receptors and weaken the cough reflex. It can relax the spastic bronchial smooth muscle, reduce airway resistance, and is suitable for inhalation and local drug administration in the respiratory tract. The raw material of pentoxyverine citrate has a purity higher than 99%. After recrystallization, it is fully dried and ground. Its macroscopic appearance is white powder. The microscopic appearance of the scanning electron microscope is blocky particles. Scanning electron microscope microscopic appearance as image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com