Method for producing epoxypropane through reaction between cumyl hydroperoxide and propylene
A technology of cumene hydroperoxide and propylene oxide, which is applied in the production of bulk chemicals, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of poor reaction stability, low selectivity of product propylene oxide, and catalyst Low activity and other problems, to achieve the effect of reducing quantity, improving catalytic performance and increasing hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
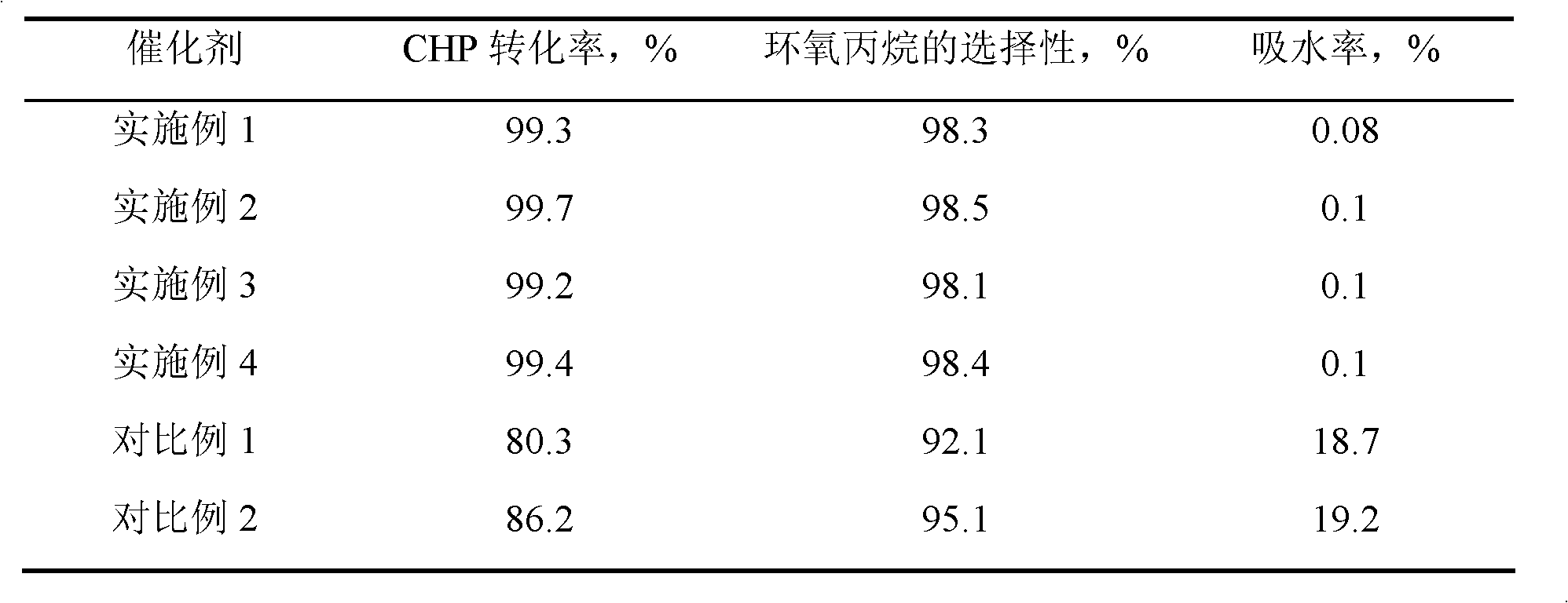
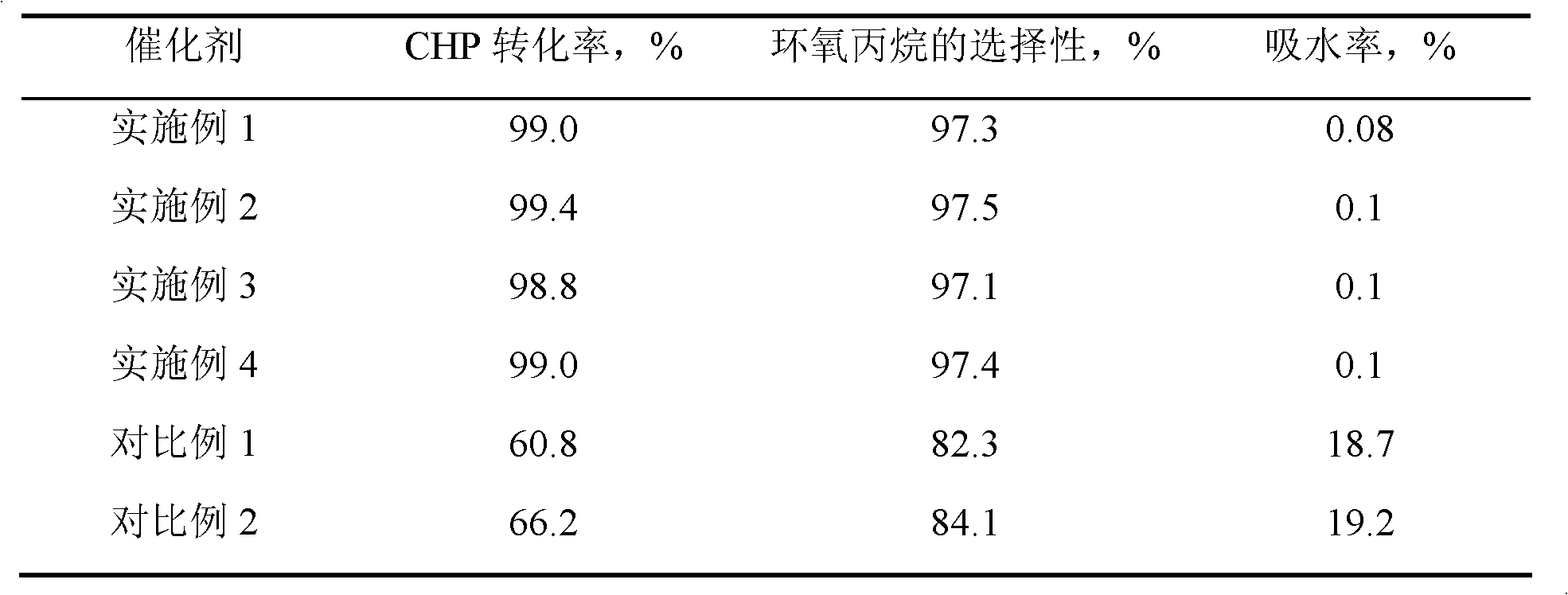
Examples
Embodiment 1
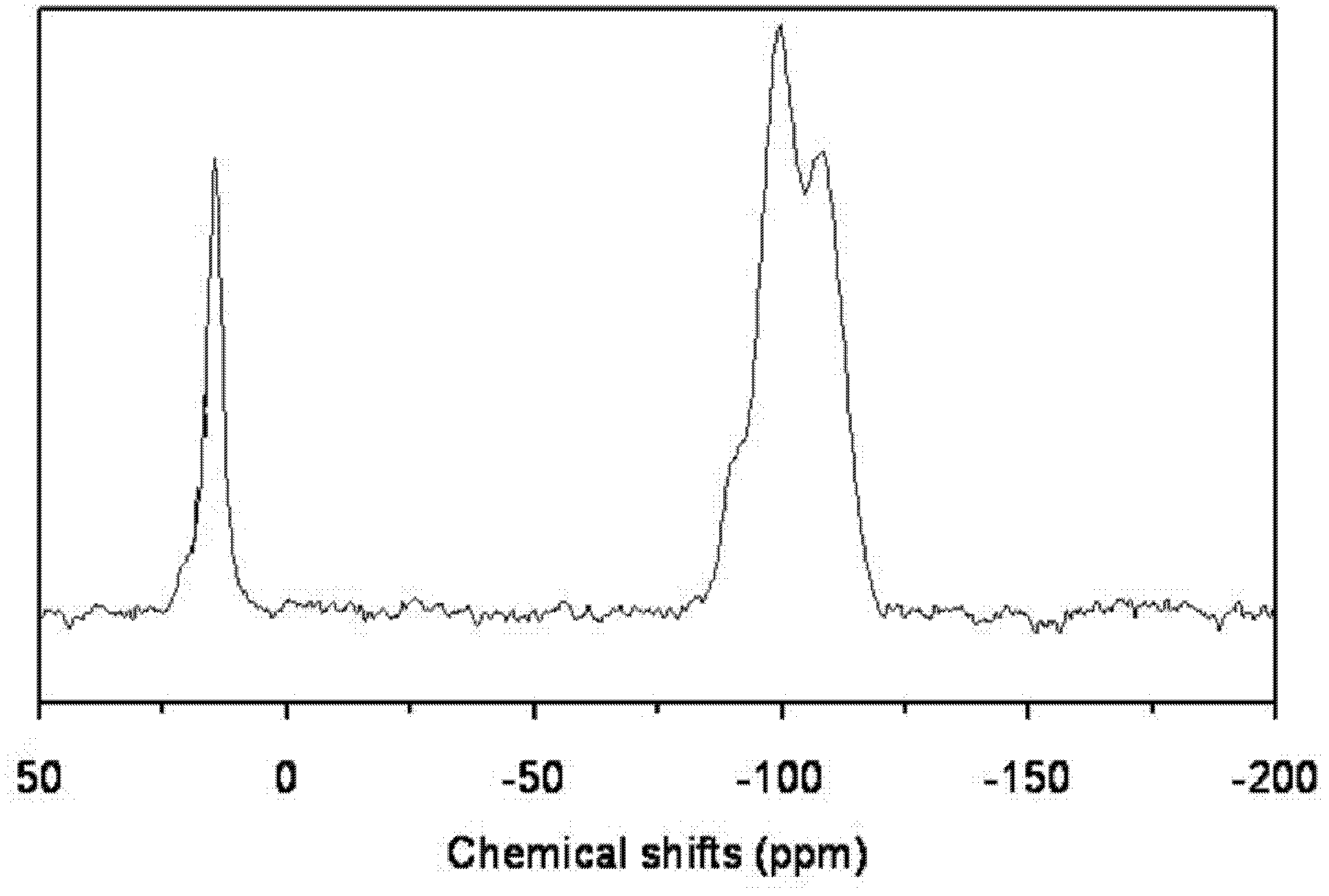
[0015] 7.23 g of n-hexadecylamine was put into a solution consisting of 63 g of deionized water and 32.2 g of ethanol, stirred and dissolved at 50° C. to form solution A. 1 mole of methyltrimethoxysilane and 0.68 g of butyl titanate were dropped into a mixed solution consisting of 6 g of isopropanol and 19 moles of ethyl orthosilicate, and stirred for 30 minutes to form solution B. Pour solution B into solution A, stir for 18 hours, filter, wash and dry, and bake at 350° C. for 8 hours to obtain precursor I. Precursor I was then placed in a quartz tubular reactor at a temperature of 100° C. under a nitrogen atmosphere, passed through N-trimethylsilyl imidazole to react for 1.5 hours, and then purged for 2 hours under a nitrogen atmosphere to obtain HMS structure titanium silicon molecular sieve. Wherein, the molar ratio of each component in the raw material is: methyltrimethoxysilane: ethyl orthosilicate: butyl titanate: hexadecylamine: water = 1: 19: 0.002: 0.03: 3.5, hexame...
Embodiment 2
[0017]Same as [Example 1], except that the molar ratio of each component in the raw material is: methyltrimethoxysilane: ethyl orthosilicate: butyl titanate: hexadecylamine: water=1: 19: 0.004: 0.04: 3.5 , under nitrogen atmosphere, the reaction temperature of precursor I and hexamethyldisilazane is 200°C, the reaction time is 3 hours, and the weight ratio of hexamethyldisilazane to precursor I is 0.05. The obtained product 29 Si CP / MAS NMR spectrogram is similar to [Example 1].
Embodiment 3
[0019] Same as [Example 1], except that the molar ratio of each component in the raw material is: methyltrimethoxysilane: ethyl orthosilicate: butyl titanate: octadecylamine: water=1: 5: 0.003: 0.04: 4.5 , under a nitrogen atmosphere, the reaction temperature of precursor I and hexamethyldisilazane is 250°C, the reaction time is 5 hours, and the weight ratio of N,0-bistrimethylsilylacetamide to precursor I is 0.005. The obtained product 29 Si CP / MAS NMR spectrogram is similar to [Example 1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com