Inhalation aerosol containing anticholinergic drug, preparation process and application method of inhalation aerosol
An aerosol and antistatic agent technology, which is applied in the field of inhalation aerosols containing anticholinergic drugs, can solve the problems of low drug deposition rate in the lungs, less than expected curative effect, and poor reproducibility of drug treatment. Achieve the effects of reducing systemic adverse reactions, improving drug stability, and improving drug compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
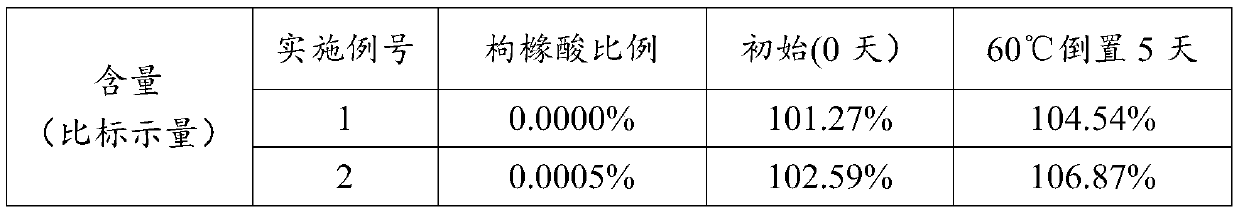
Embodiment 1~6
[0043] Inhalation Aerosols Containing Anticholinergic Drugs:
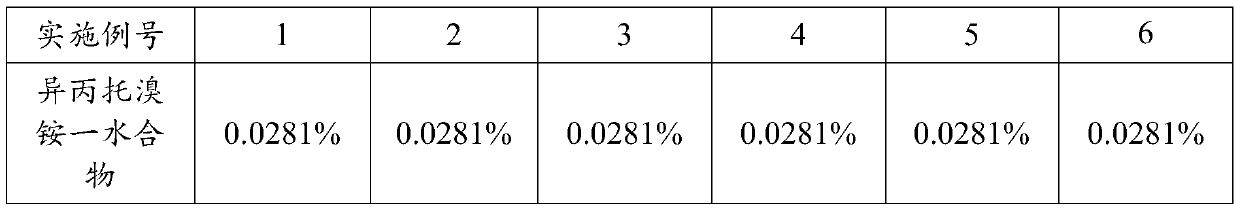
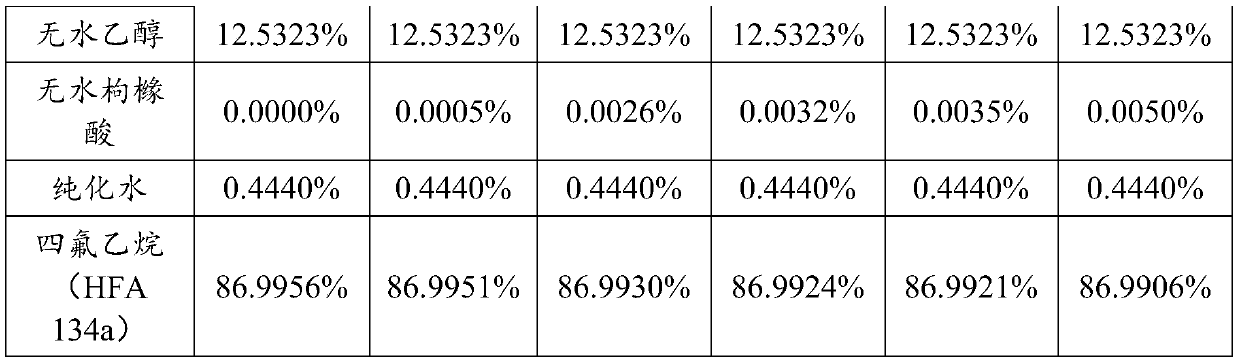
[0044]The addition amount of active ingredients is 0.0281% (w / w), the addition amount of anhydrous ethanol is 12.5323% (w / w), the addition amount of anhydrous citric acid is 0.0000% to 0.0050% (w / w), and the addition amount of purified water is 0.4440% (w / w), and the amount of propellant added is 86.9906% to 86.9956% (w / w), as shown in Table 1 below:
[0045] Table 1. Formulations (w / w) of Examples 1 to 6
[0046]
[0047]
[0048] The preparation method is:
[0049] One-step pressure filling is adopted, and the specific steps are as follows:
[0050] (a) Use suitable solvents such as ethanol and blank propellant to clean and disinfect the preparation pot and pipeline;
[0051] (b) take by weighing the ipratropium bromide of recipe quantity, add the dehydrated alcohol that is about recipe quantity 15% (w / w), stir and dissolve;
[0052] (c) take by weighing anhydrous citric acid, add the purified water of ...
Embodiment 7~10
[0065] Inhalation Aerosols Containing Anticholinergic Drugs:
[0066] The dosage of active ingredients is 0.0279% (w / w), the dosage of absolute ethanol is 12.9024% (w / w), the dosage of oleic acid is 0.0000%~0.1500% (w / w), and the dosage of purified water is 0.2587% (w / w), the amount of propellant added is 86.6610% to 86.8110% (w / w), see Table 4 for details:
[0067] Table 4. Formulations (w / w) of Examples 7-10
[0068] Example number 7 8 9 10 ipratropium bromide monohydrate 0.0279% 0.0279% 0.0279% 0.0279% anhydrous ethanol 12.9024% 12.9024% 12.9024% 12.9024% Oleic acid 0.0000% 0.0126% 0.1009% 0.1500% purified water 0.2587% 0.2587% 0.2587% 0.2587% Tetrafluoroethane (HFA 134a) 86.8110% 86.7984% 86.7101% 86.6610%
[0069] The preparation method is:
[0070] One-step pressure filling is adopted, and the specific steps are as follows:
[0071] (a) Use suitable solvents such as ethanol and blank propellant to clean an...
Embodiment 11~15
[0085] Inhalation Aerosols Containing Anticholinergic Drugs:
[0086] The addition amount of active ingredients is 0.0275% (w / w), the addition amount of absolute ethanol is 10.0% to 15.0% (w / w), the addition amount of anhydrous citric acid is 0.0030% (w / w), and the addition amount of purified water is 0.3617% (w / w), and the added amount of propellant is 84.6078%-89.6078% (w / w), as shown in Table 7:
[0087] Table 7. Formulations (w / w) of Examples 11-15
[0088]
[0089]
[0090] The preparation method is:
[0091] One-step pressure filling is adopted, and the specific steps are as follows:
[0092] (a) Use suitable solvents such as ethanol and blank propellant to clean and disinfect the preparation pot and pipeline;
[0093] (b) take by weighing the ipratropium bromide of recipe quantity, add the dehydrated alcohol that is about recipe quantity 25% (w / w), stir and dissolve;
[0094] (c) take by weighing anhydrous citric acid, add the purified water of recipe quantity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com