Dry powder inhalant of interferon alpha
A dry powder inhaler and interferon α technology, which is applied in antiviral agents, medical preparations containing active ingredients, peptide/protein components, etc., can solve the problems of increasing the complexity and cost of the preparation process, the stability of dry powder inhalers, and the use of performance impact etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of the dry powder inhalation of interferon alpha
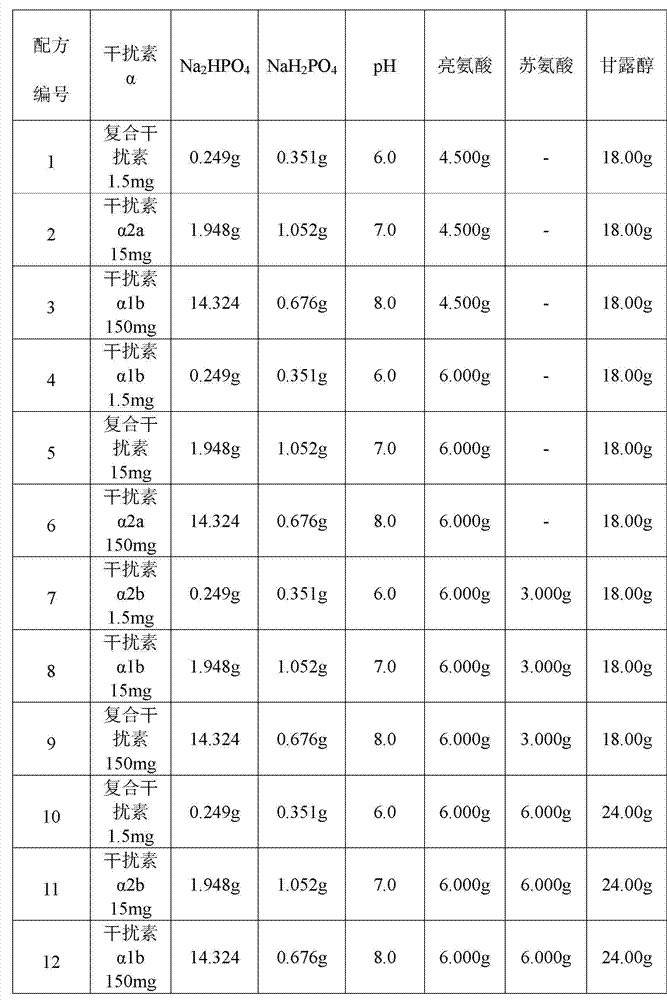
[0031] Prepare each solution for spray drying according to the formula in Table 1 below, and then use Buchi B-290 spray dryer to spray dry according to the following conditions: spray drying air inlet temperature 120 ° C, spray drying air flow rate 500 L / h, spray liquid flow rate 5ml / min, spray liquid temperature 8°C, keep spray drying gas inlet temperature and flow rate for 20 minutes after all liquid spray is completed.
[0032] Table 1 Prepare the solution formula (1000ml) for spray drying of the dry powder inhaler of interferon α
[0033]
Embodiment 2
[0034] Embodiment 2: the quality evaluation of the dry powder inhaler particle of interferon alpha
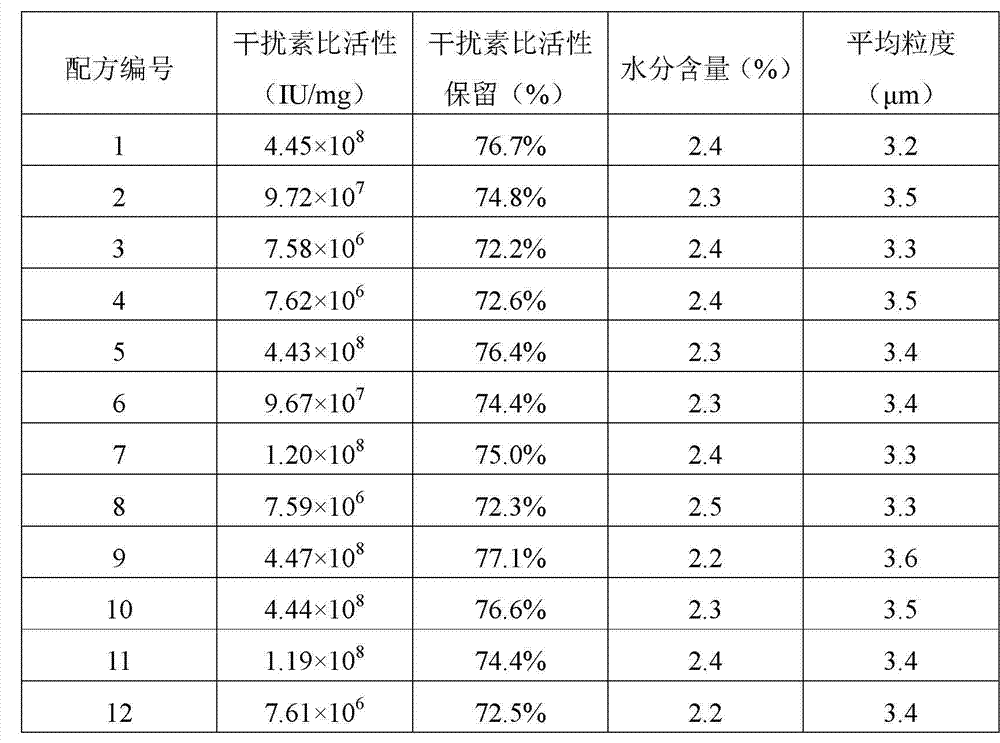
[0035] According to the provisions of the "Chinese Pharmacopoeia 2010 Edition (Part Three)" appendix "Interferon Biological Activity Assay Method", the activity of interferon α in the solution after the dissolution of the prepared interferon α dry powder inhalation particles of each formula (IU / ml); According to the provisions of the second method Lowry method of "Protein Content Determination Method" in the appendix of "Chinese Pharmacopoeia 2010 Edition (Part Three)", the interferon α in the solution of the prepared interferon α dry powder inhalation particles of each formula was determined after dissolution The concentration (mg / ml) was divided by the two to obtain the specific activity (IU / mg) of interferon α in the prepared interferon α dry powder inhalation particles of each formula. The specific activity of interferon α in each solution for spray drying was measured by ...
Embodiment 3
[0041] Embodiment 3: the in vitro deposition test of the dry powder inhalation of interferon alpha
[0042] A part of the dry powder inhalation sample of interferon alpha of each formula obtained by the method of Example 1 is subpackaged with the amount of 20 mg per capsule, respectively, to obtain non-loaded body capsule subpackage samples of formula 1-12; Mix evenly with commercially available large-size lactose carrier granules at a mass ratio of 1:2, and subpackage in an amount of 20 mg per capsule to obtain loading capsule subpackage samples of formulas 1-12. For formula 1-12, the unloaded capsule subpackage samples and the loaded capsule subpackage samples were respectively according to the "Chinese Pharmacopoeia 2010 Edition (Part Two)" appendix "Appendix X H inhalation gas (powder) aerosol effective part drug deposition method "Determination of in vitro deposition properties. The secondary deposits were weighed and completely dissolved respectively, and then the conce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com