Patents
Literature
171results about How to "Reduce local pressure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
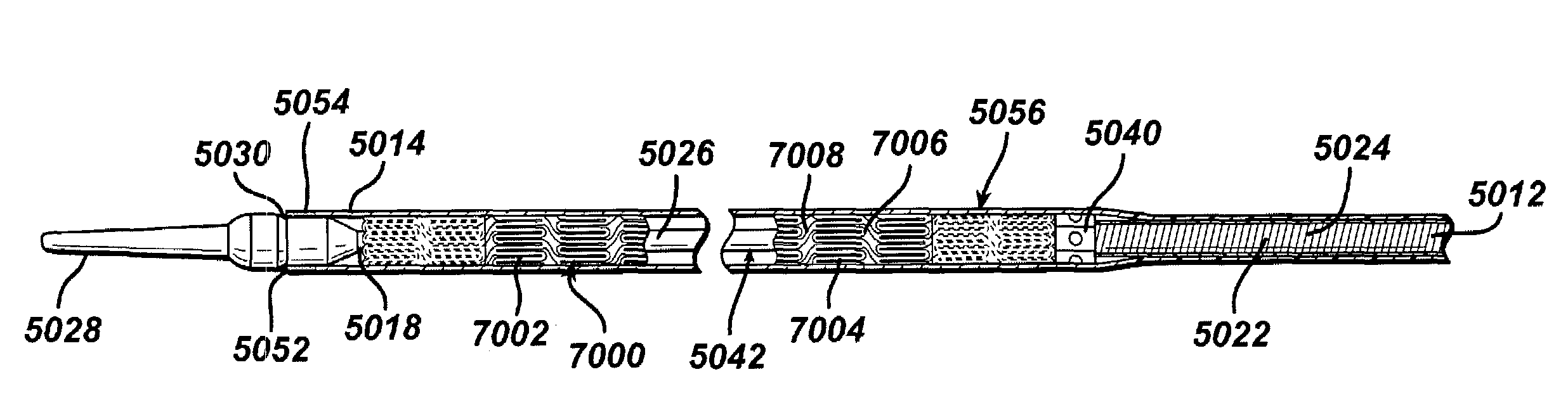
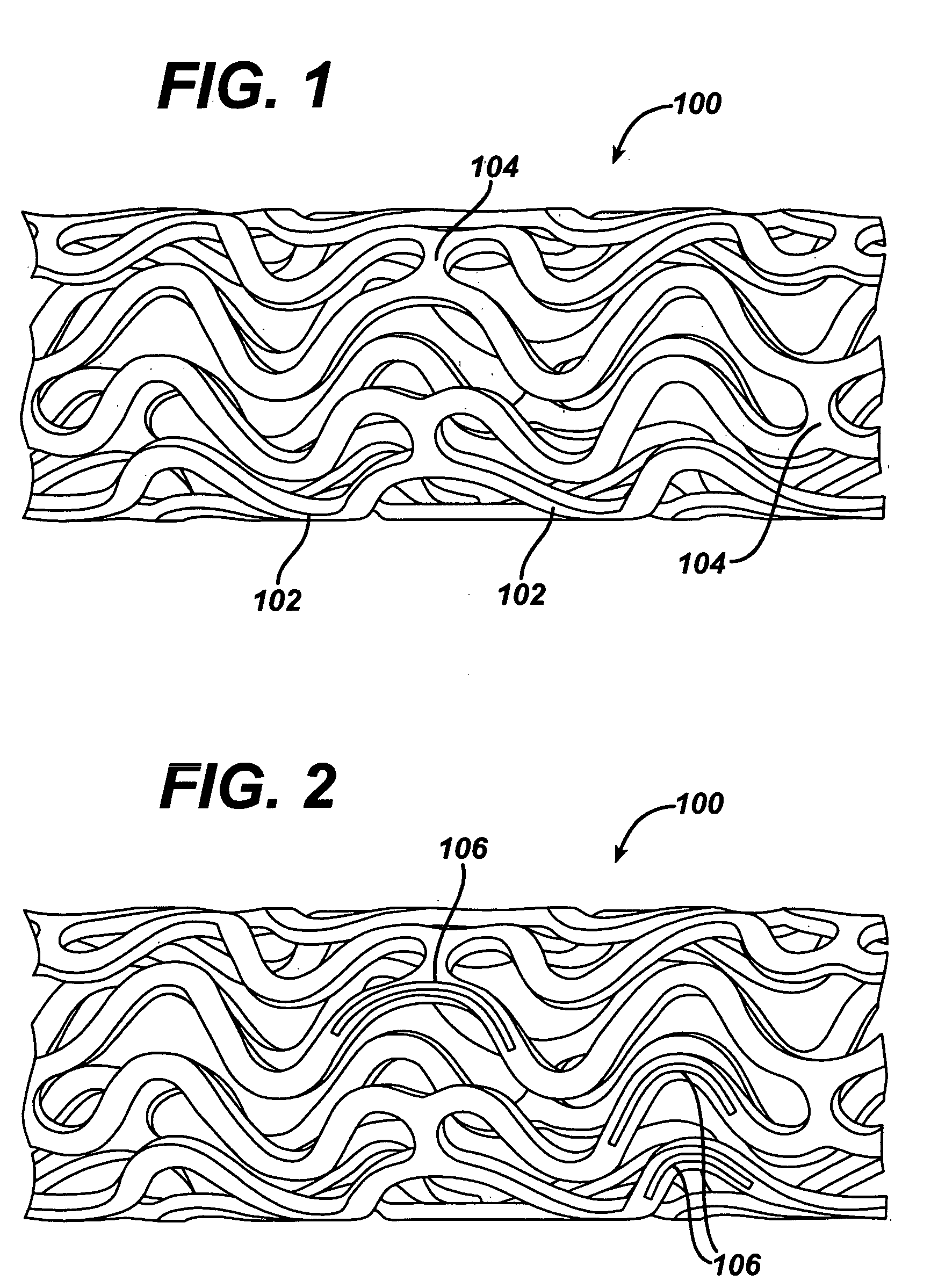
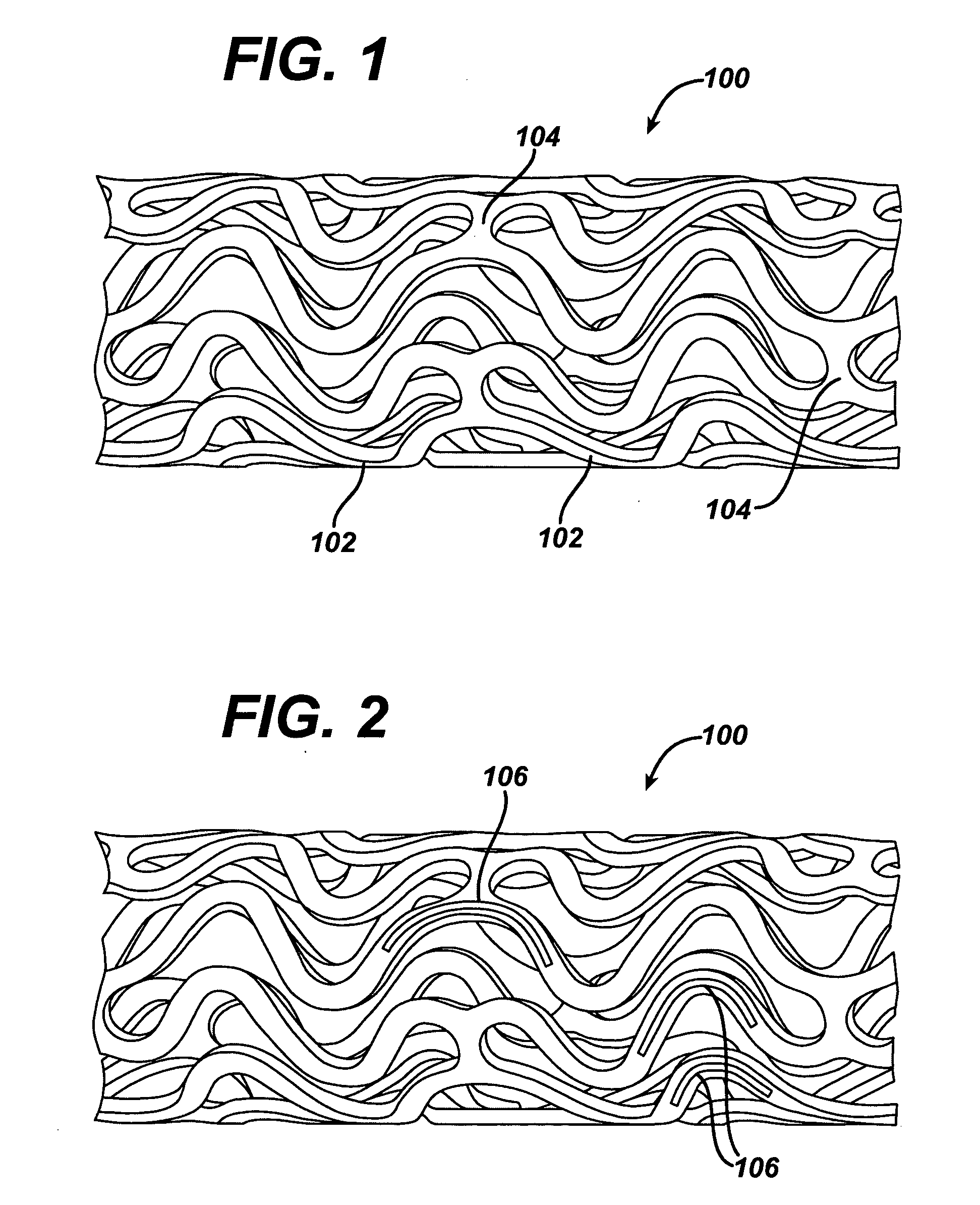
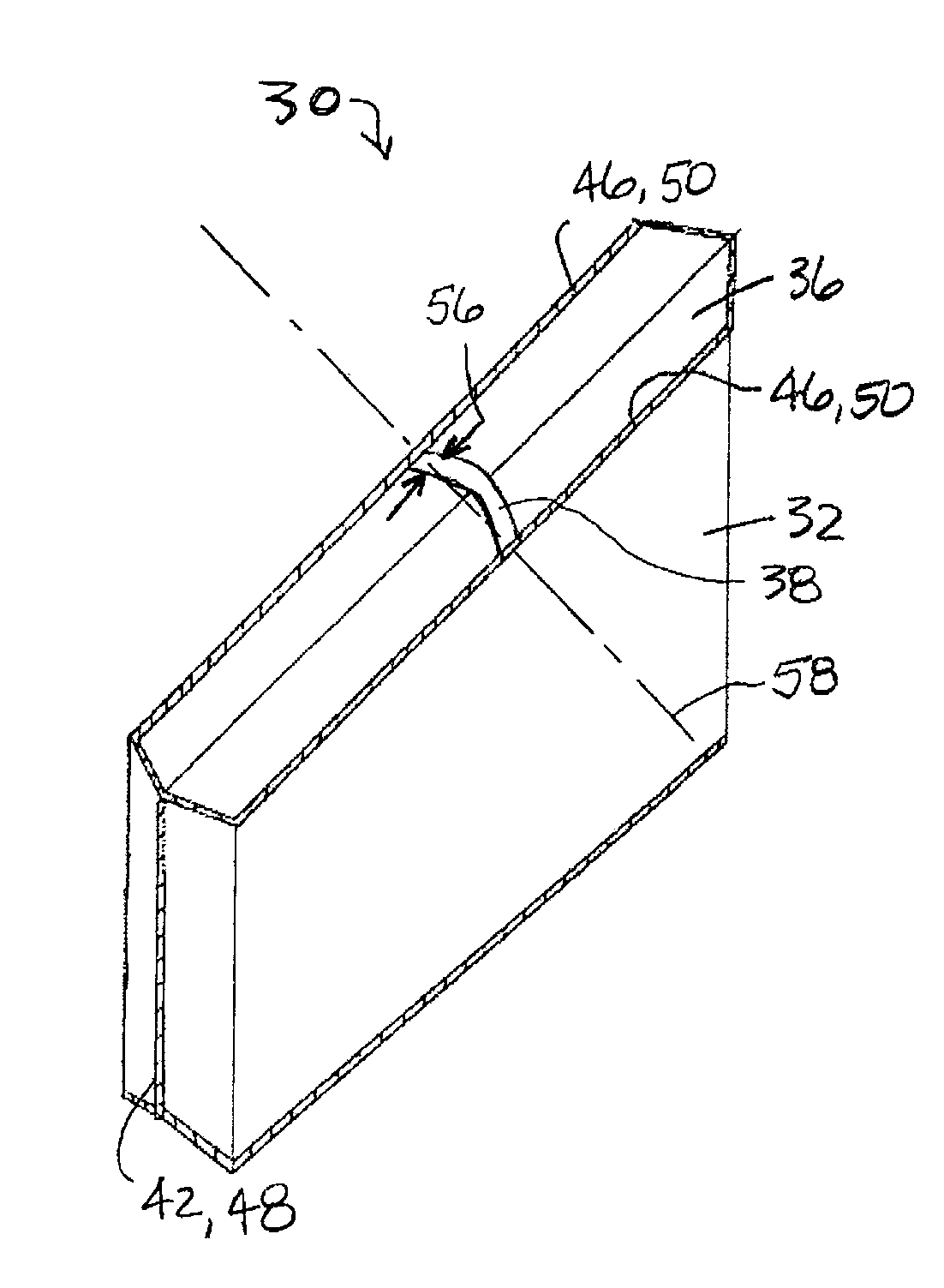
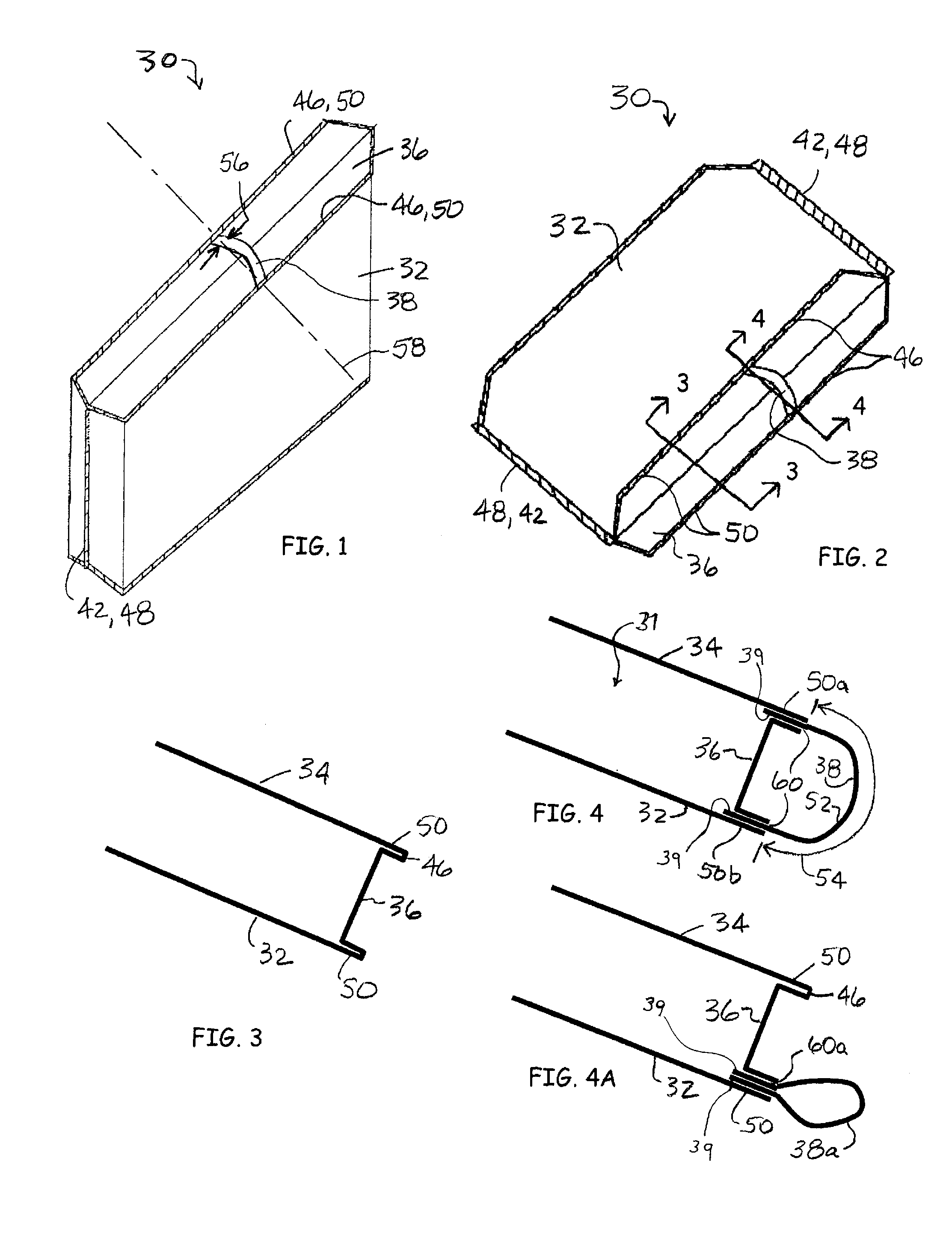
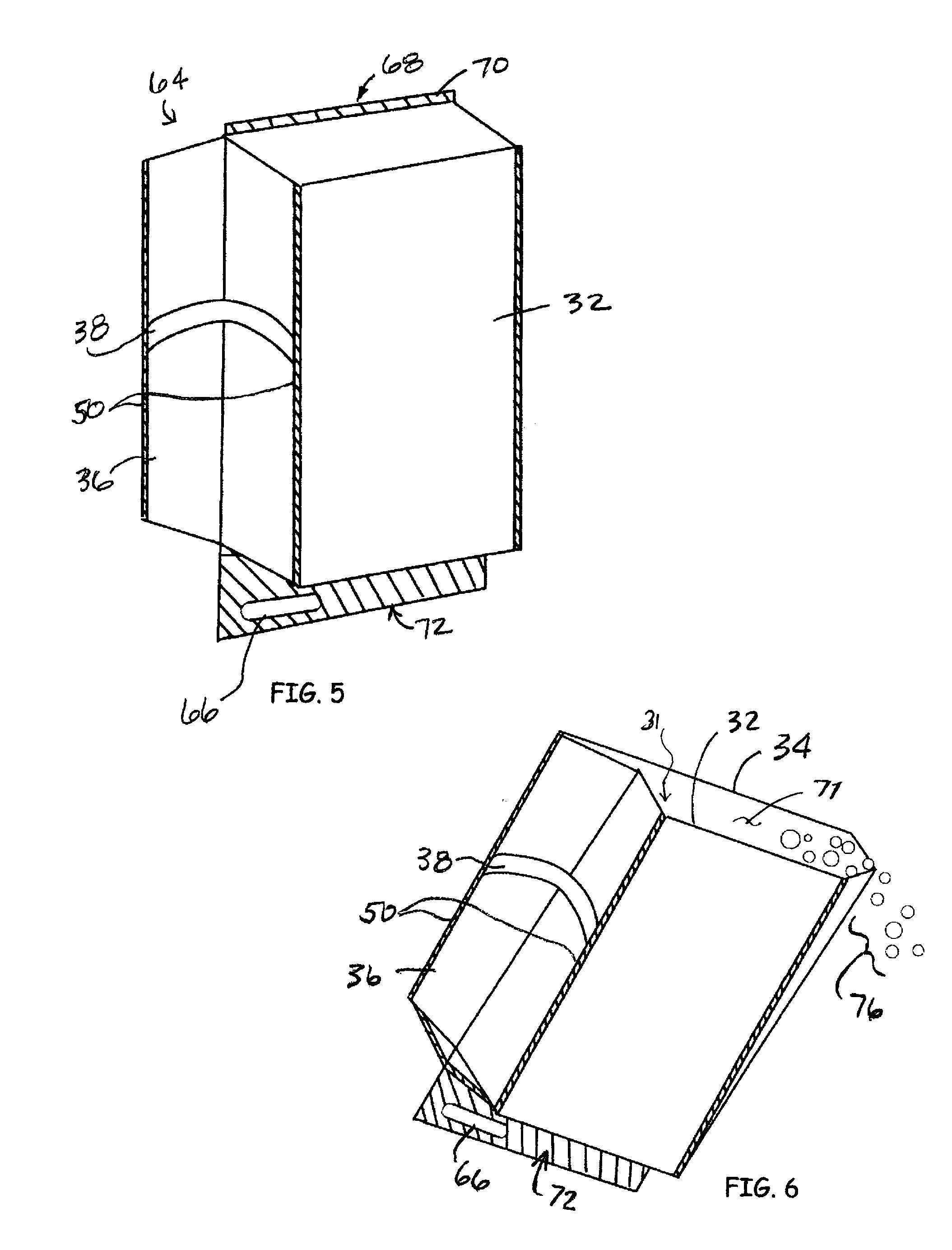
Modified delivery device for coated medical devices
InactiveUS7527632B2Minimize potential risk of damageReduce frictionStentsEar treatmentBiological bodyMedical device
Medical devices, and in particular implantable medical devices, including self-expanding stents, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
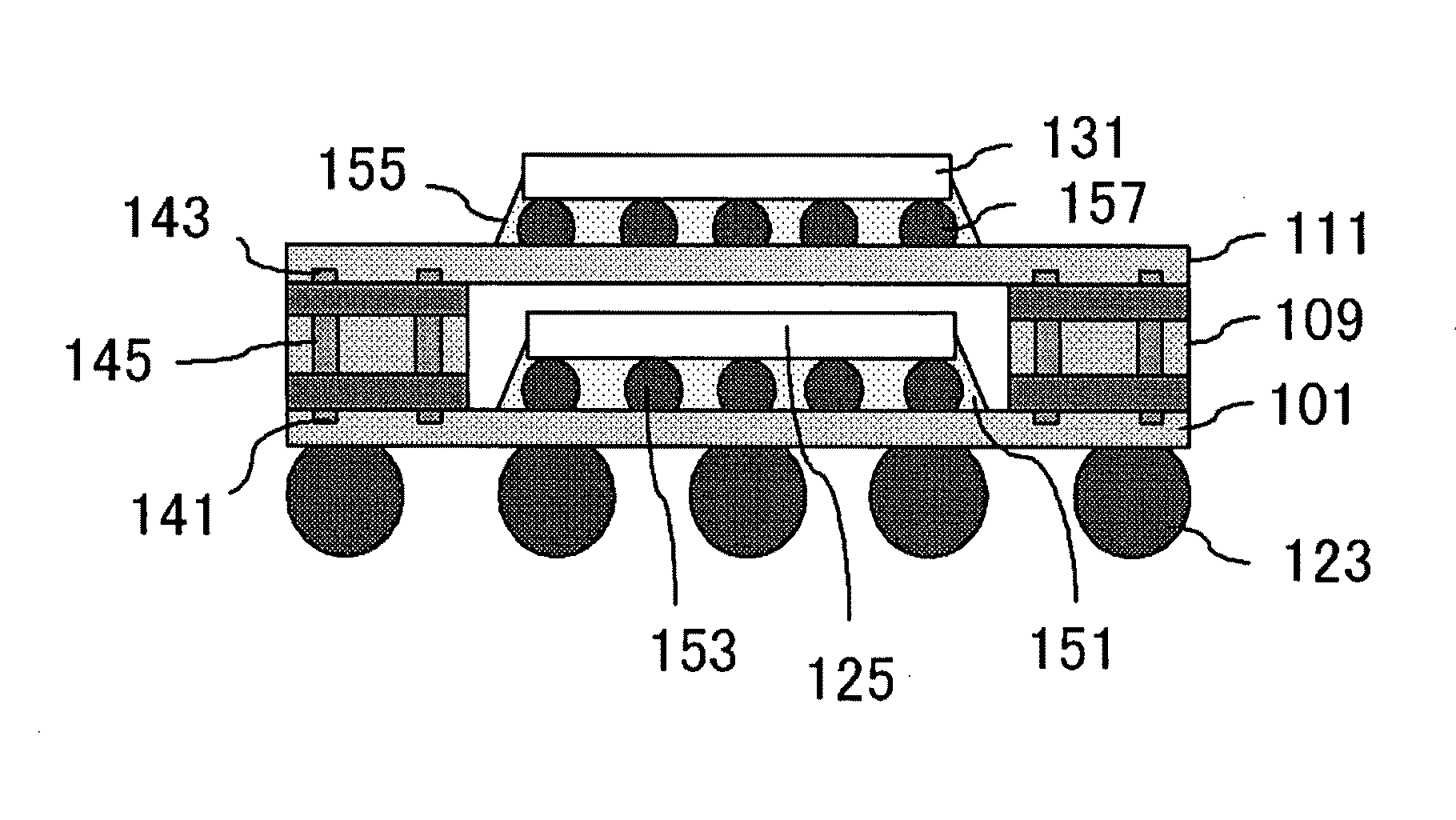
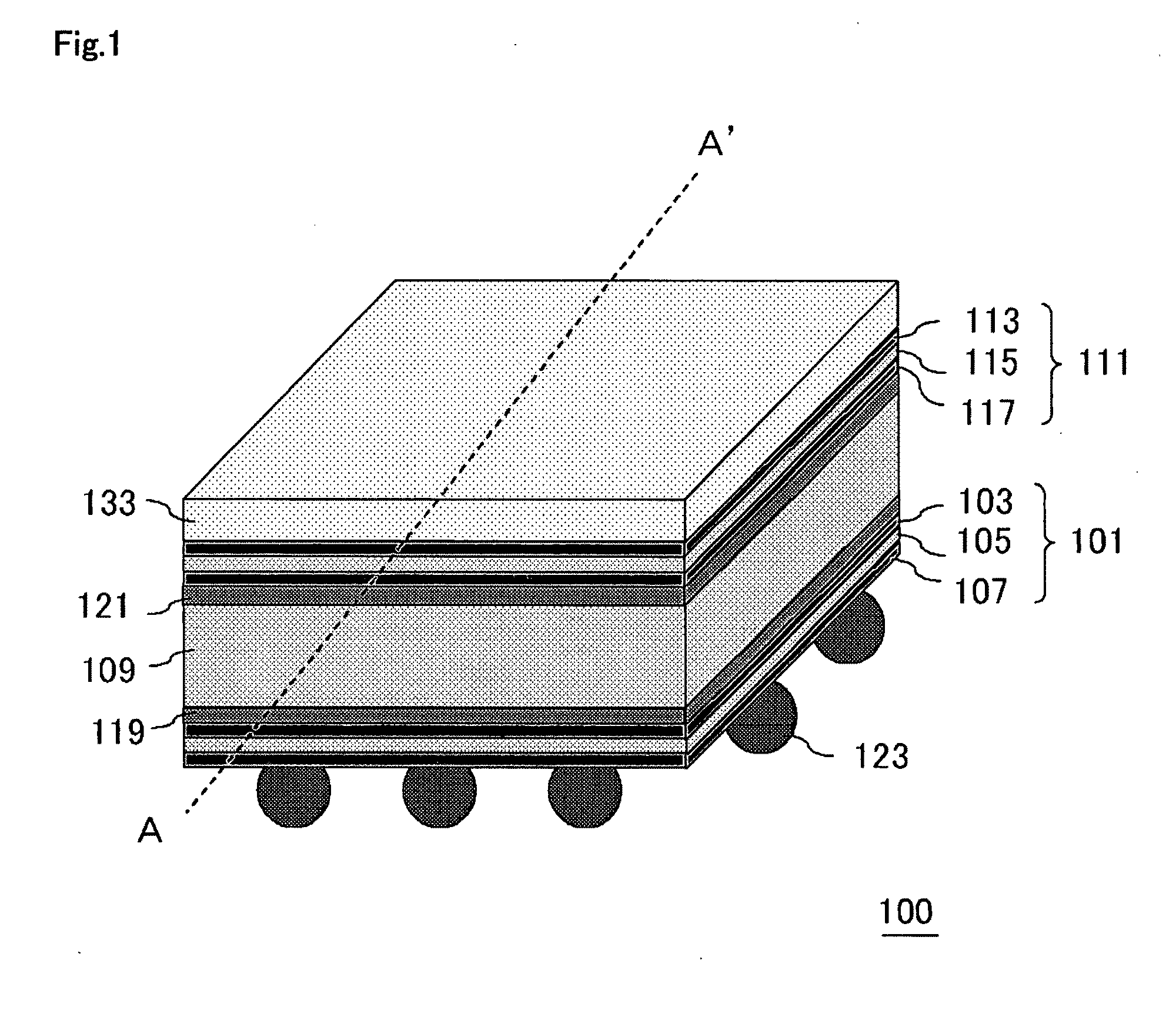
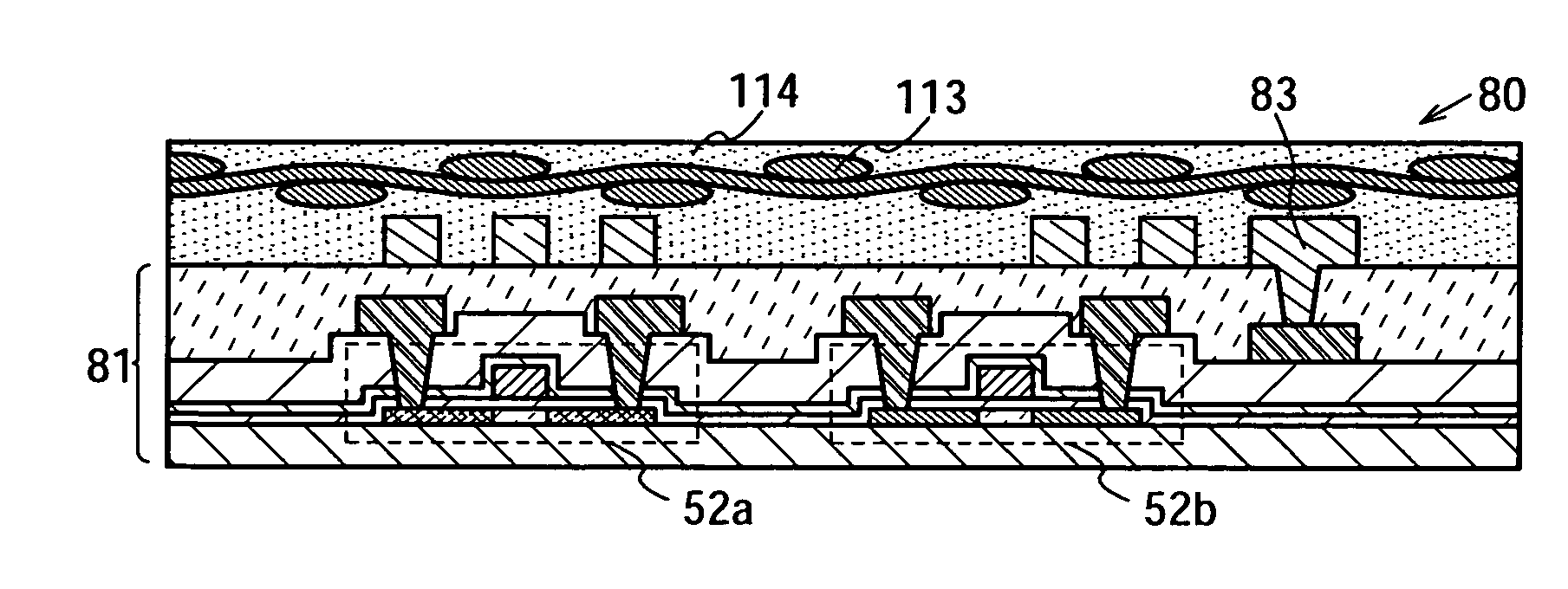
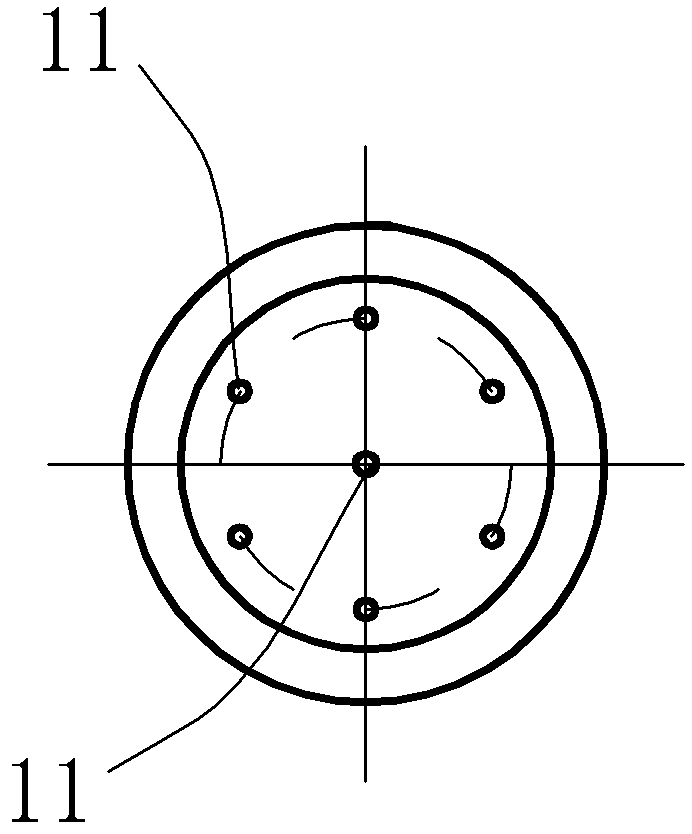
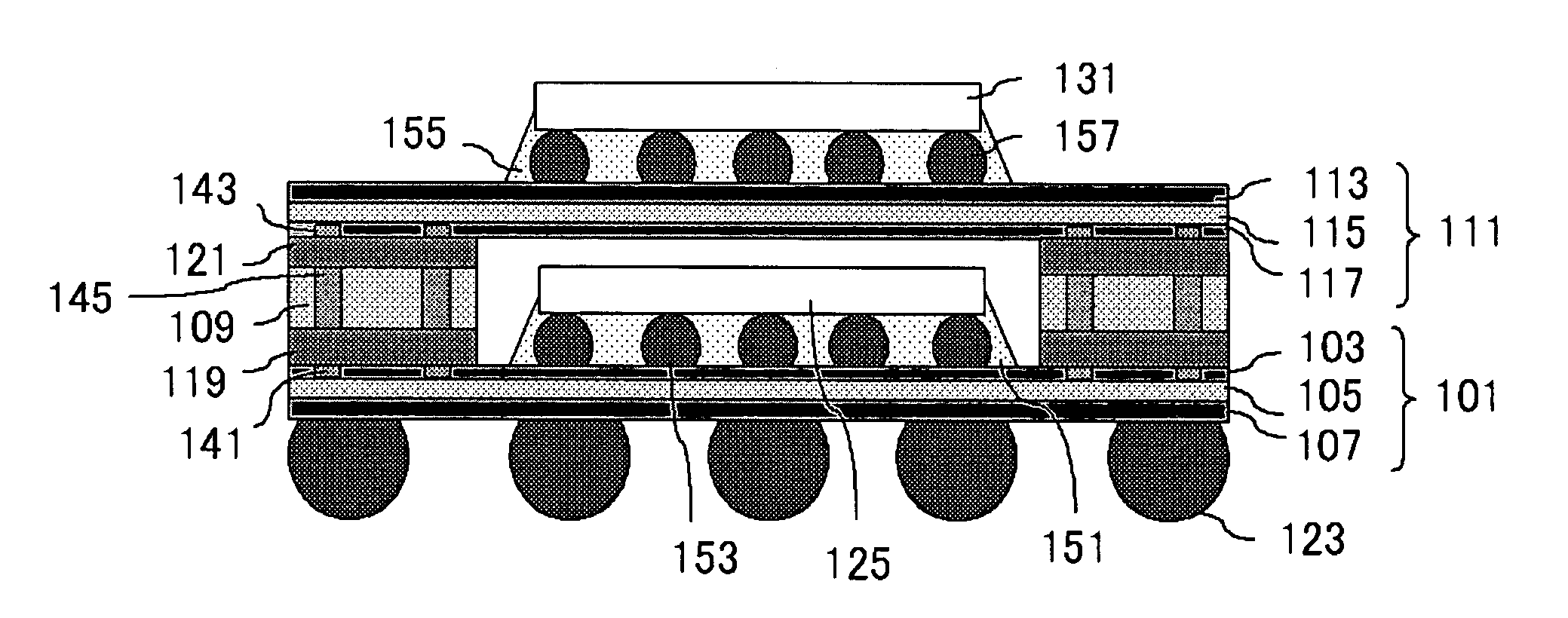
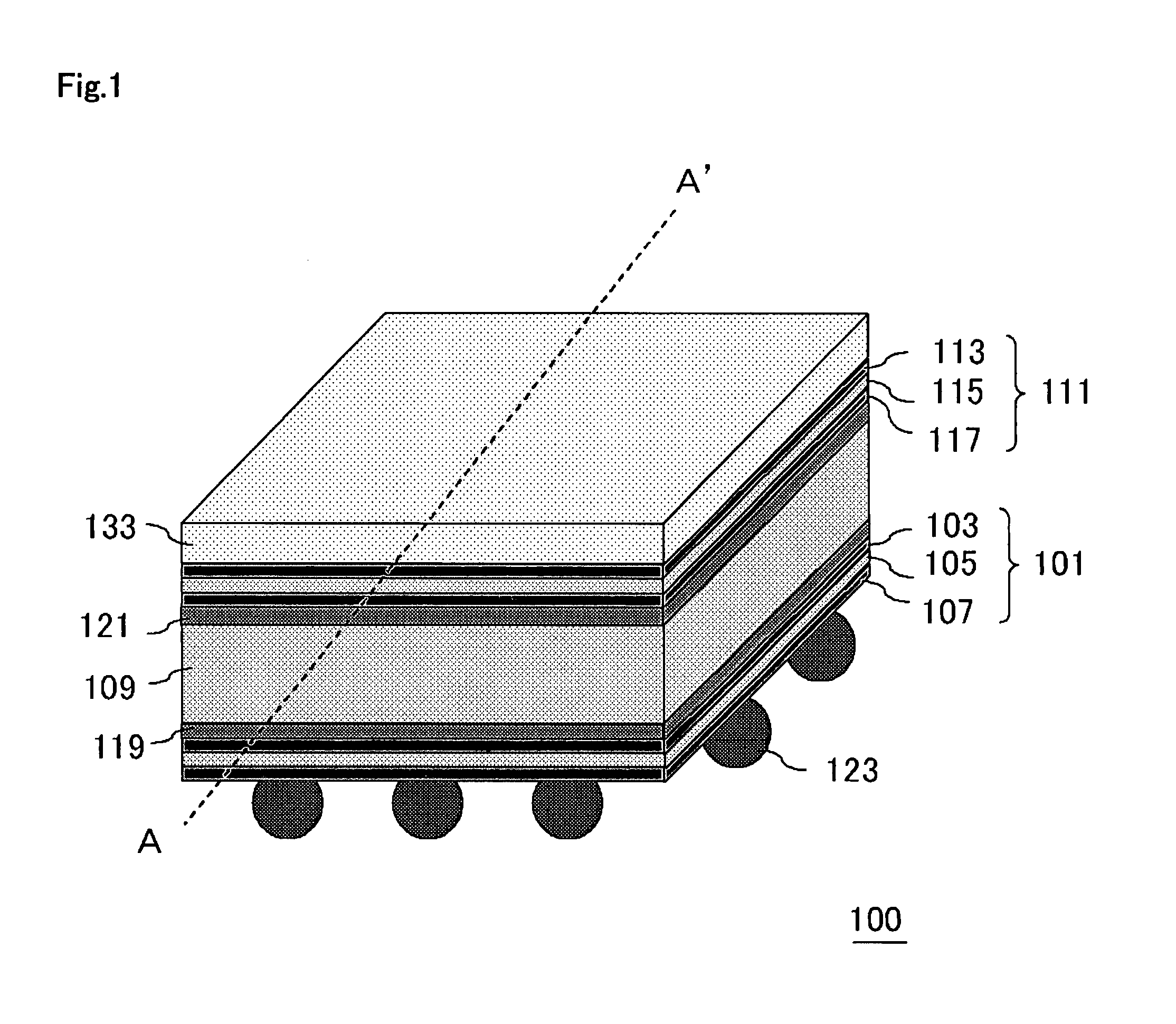
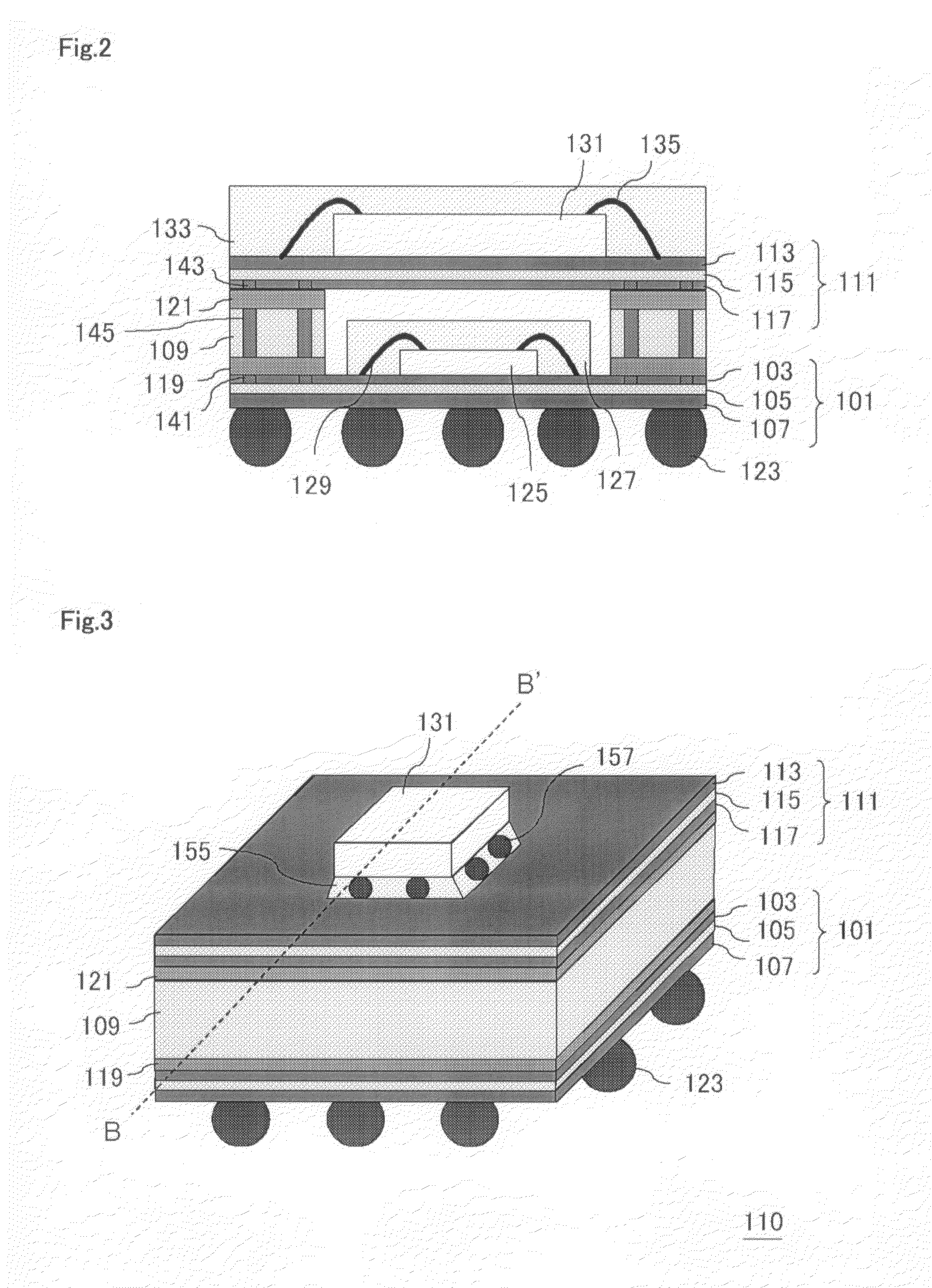
Semiconductor Device and Method for Manufacturing Semiconductor Device
InactiveUS20090243065A1Improve joint reliabilityLower elastic modulusSemiconductor/solid-state device detailsSolid-state devicesSurface mountingSemiconductor chip
A semiconductor device (100) comprises a first resin substrate (101) on which a first semiconductor chip (125) is mounted a surface thereof; a second resin substrate (111) on which a second semiconductor chip (131) is mounted on a surface thereof; and a resin base material (109), joined to a front surface of the first resin substrate (101) and to a back surface of the second resin substrate (111), so that these surfaces are electrically connected. The resin base material (109) is disposed in a circumference of the first resin substrate (101) in the surface of the first resin substrate (101). Further, the first semiconductor chip (125) is disposed in a space section provided among the first resin substrate (101), the second resin substrate (111) and the resin base material (109) in the surface of the first resin substrate (101).
Owner:SUMITOMO BAKELITE CO LTD
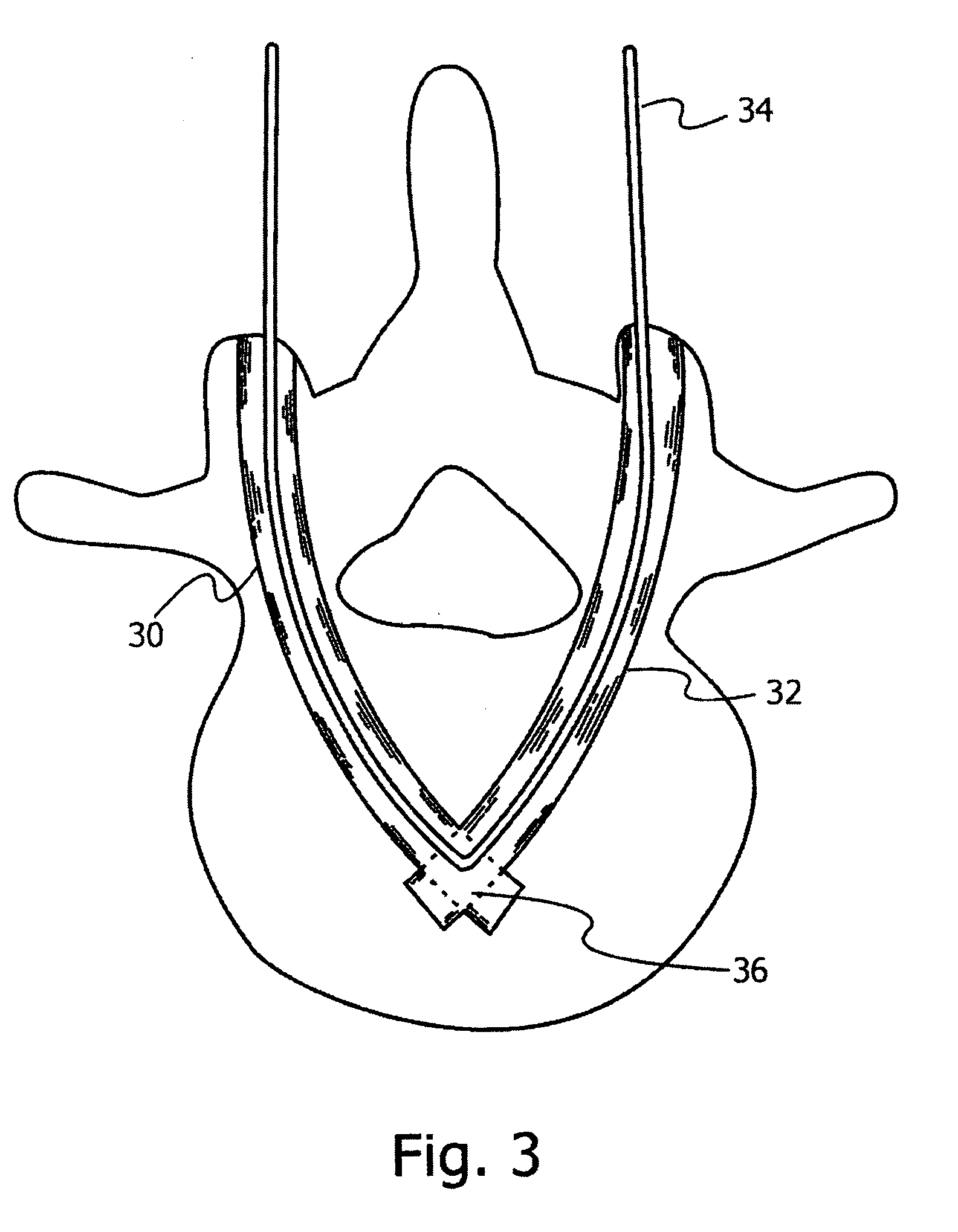
Bone anchoring system
InactiveUS20100305700A1Easy to deployReduces pull-out problemInternal osteosythesisJoint implantsIntervertebral spaceBone anchor
Methods and apparatus for connecting to a bone, which avoid the use of bone screws. A triangular shaped modular implant is used, with two sides of the structure and their respective vertex secured generally within the bone, with the base of the triangle outside the bone. These two arms, whether straight or arcuate, are cannulated, and are held together at their distal ends by a tightened cable that runs through both of the arms. The proximal ends of these arms are connected to a base side that completes the triangular structure. The device may be inserted into a vertebra and the base used for vertebral fusion. Alternatively, the arms themselves may be used to stabilize and fixate adjacent vertebrae, by insertion through adjacent vertebrae trans-segmentally. In the latter case, the vertex may be within the intervertebral space or within the vertebral body close to the intervertebral space.
Owner:SCORPION SURGICAL TECH
Substrate for producing a soldering connection
InactiveUS7271484B2Improve reliabilityReducing in fashionPrinted circuit assemblingSemiconductor/solid-state device detailsSolder maskEngineering
A solderable device includes a substrate and a soldering pad overlying the substrate. A solder mask overlies the substrate and portions of the soldering pad. The solder mask has an opening that exposes a portion of the soldering pad. The opening has at least two edges that symmetrically overlie portions of the soldering pad.
Owner:POLARIS INNOVATIONS LTD
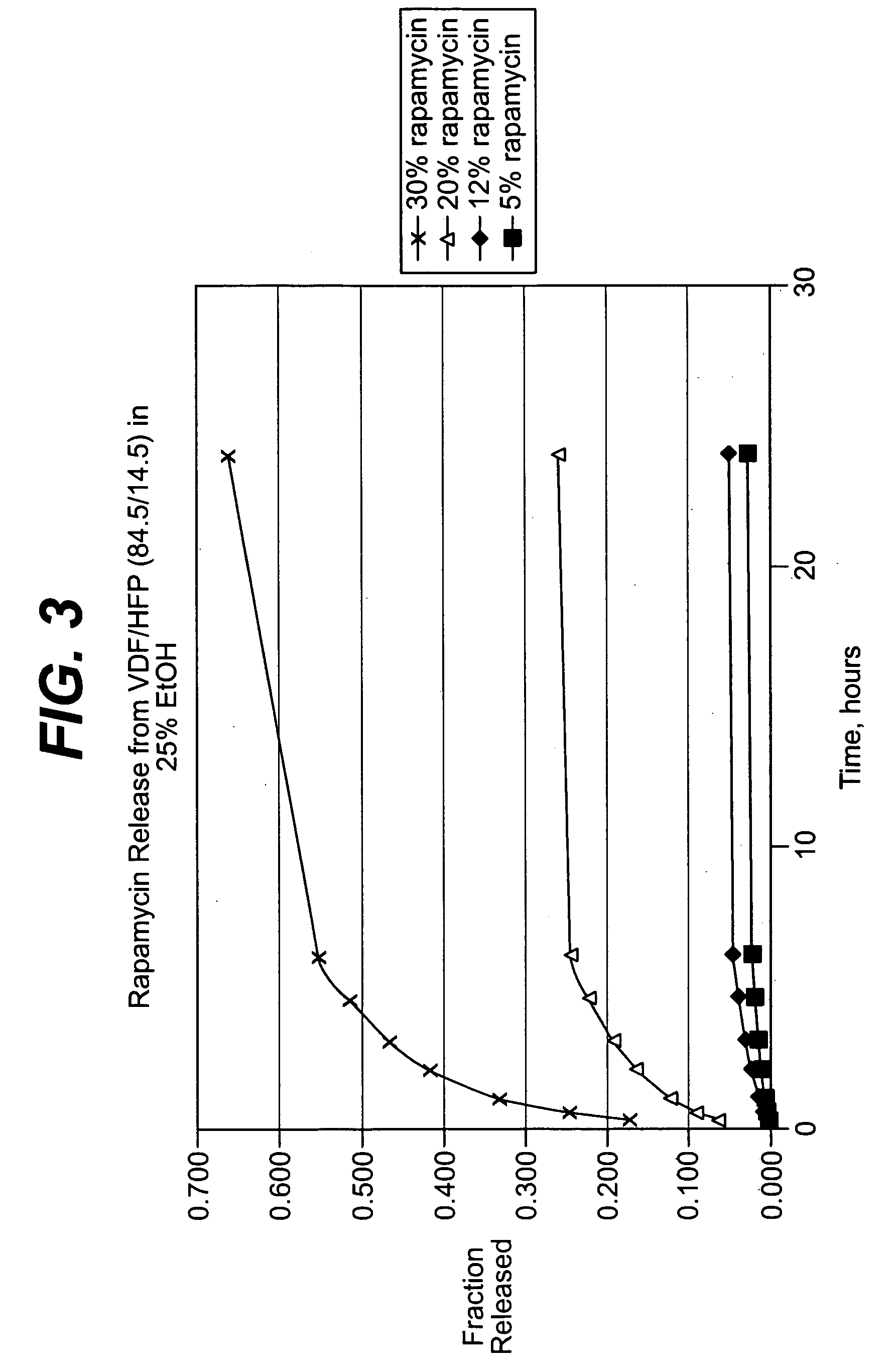
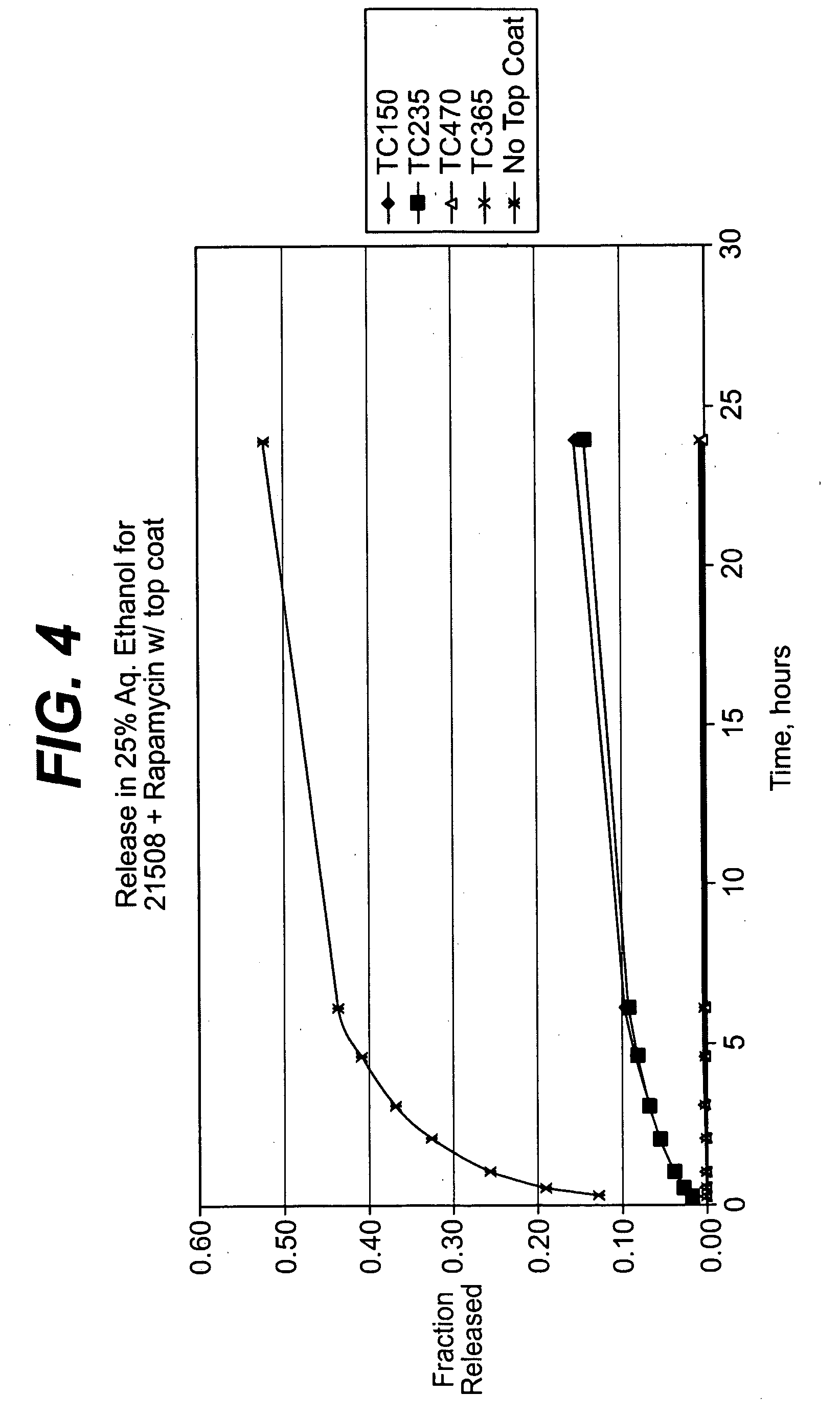
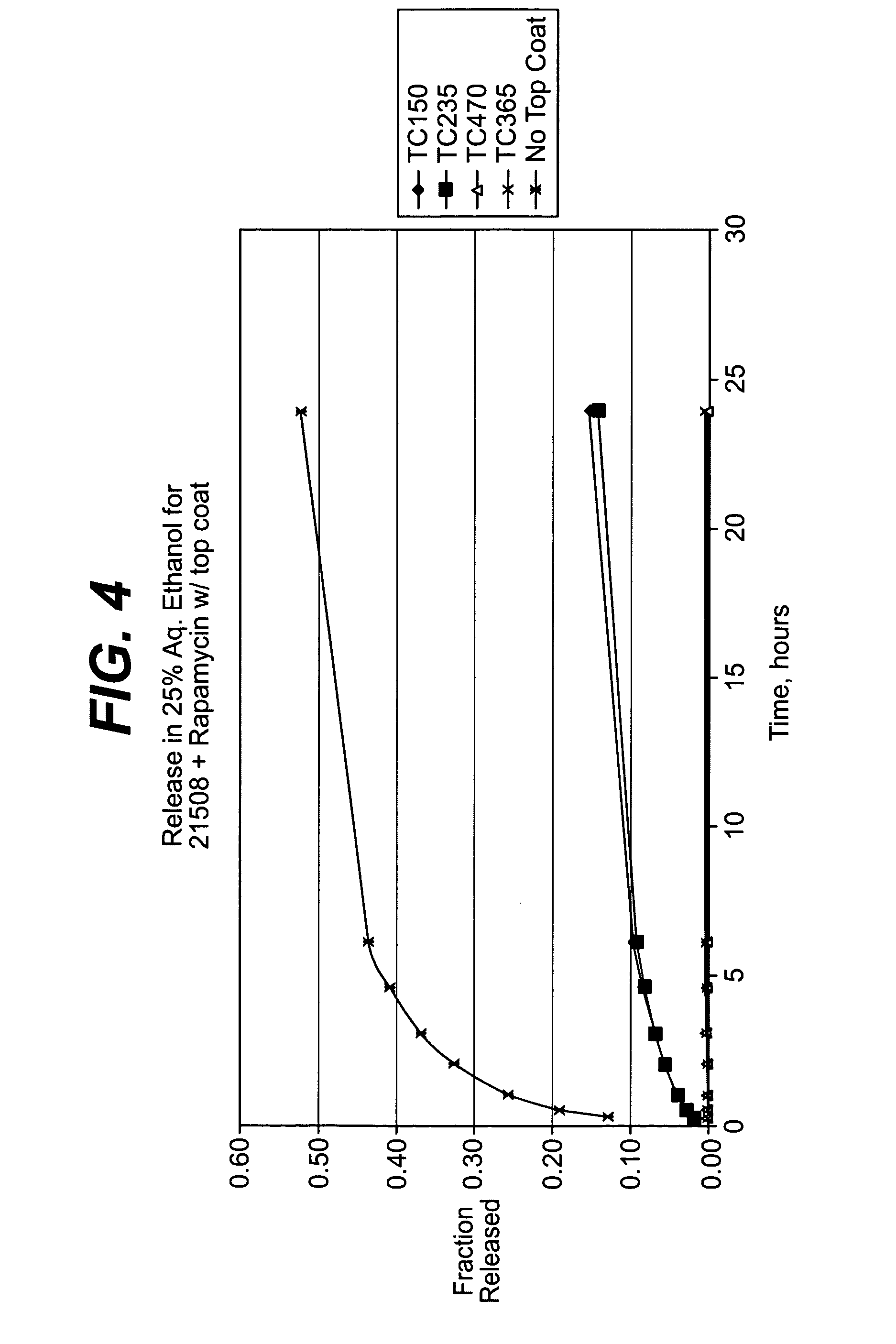
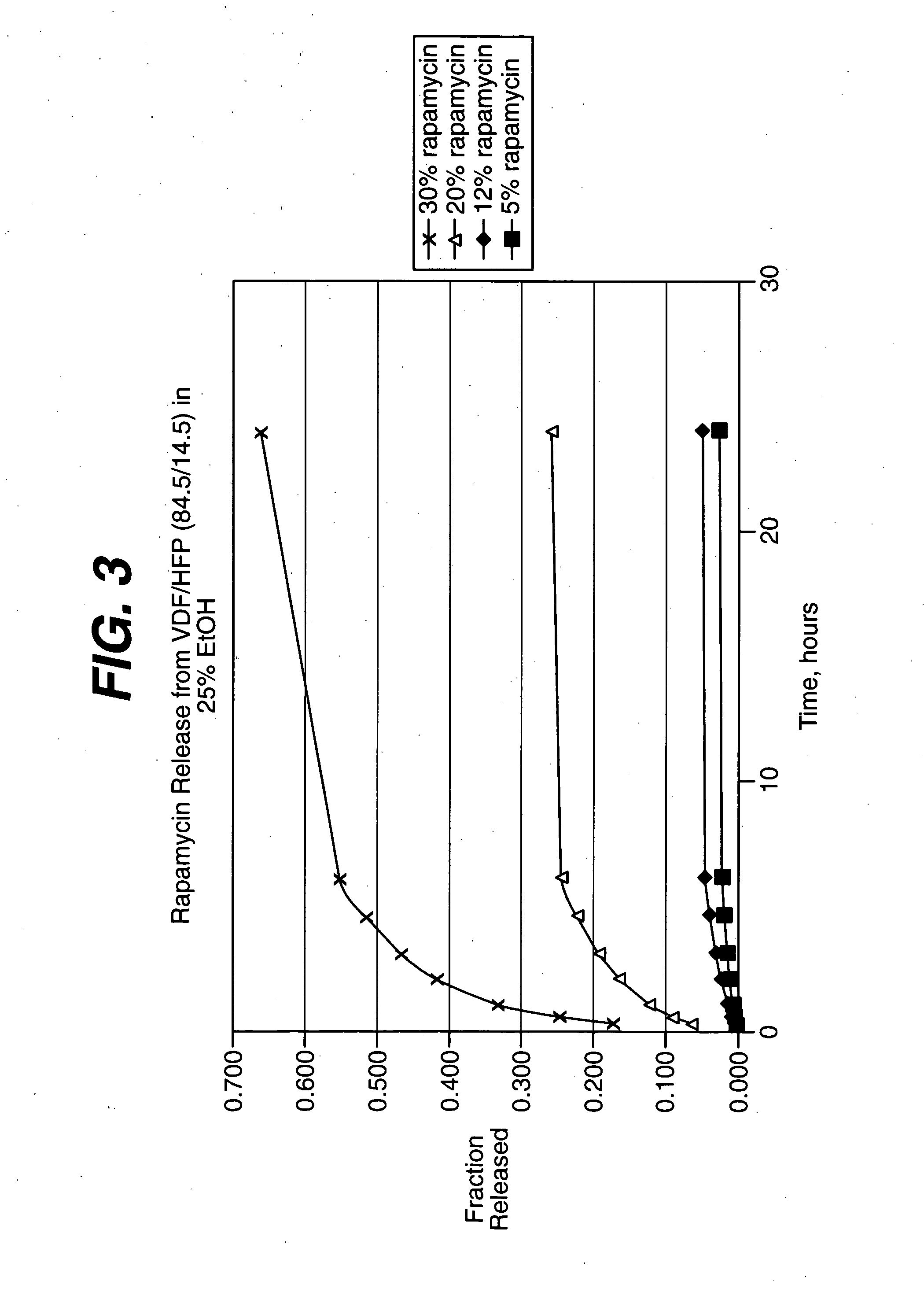
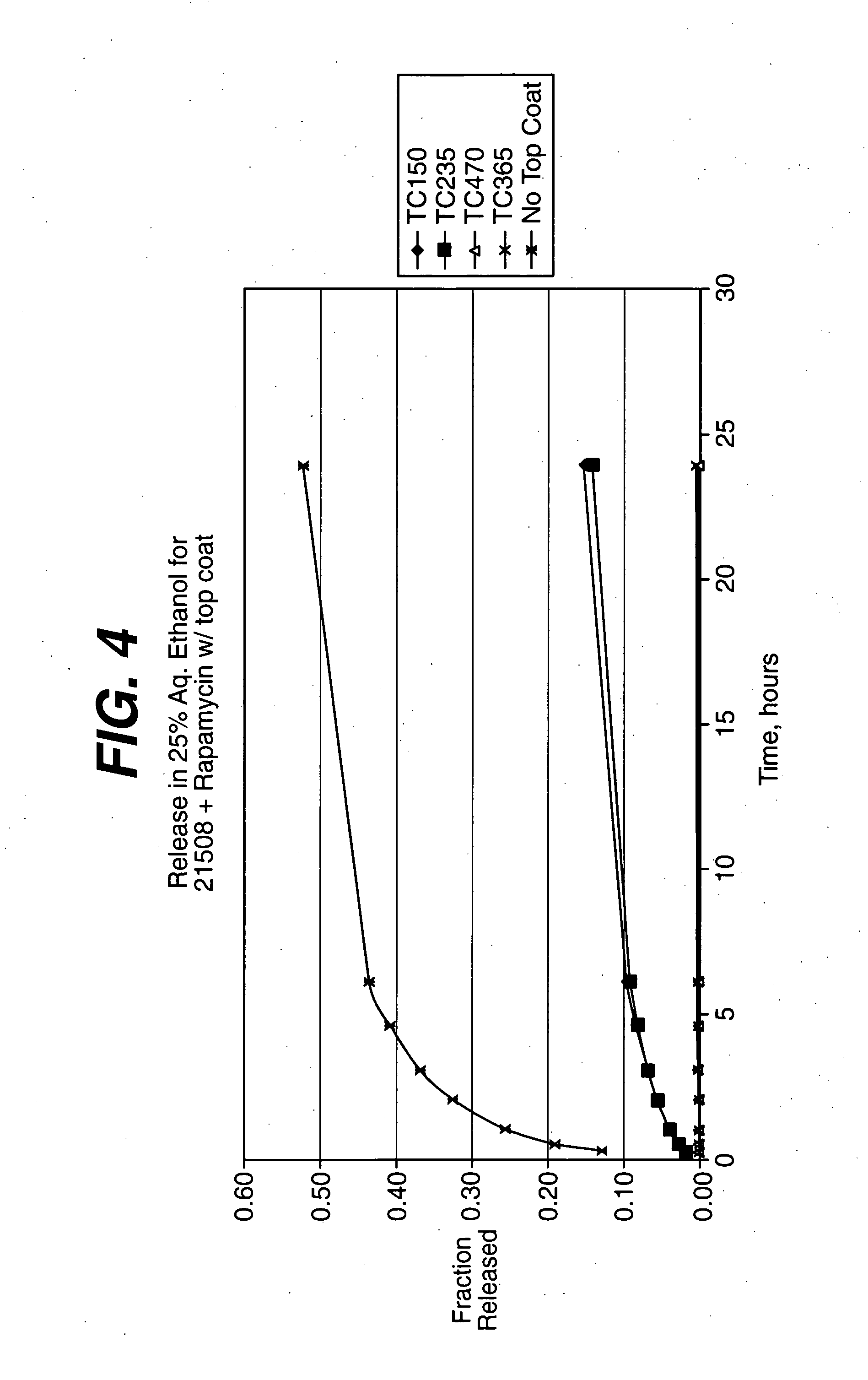
Solution formulations of sirolimus and its analogs for CAD treatment
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Injectable formulations of taxanes for cad treatment
InactiveUS20050272806A1Minimize potential risk of damageReduce frictionBiocideOrganic active ingredientsAntioxidantBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Liquid formulations, including solutions and suspensions of the various drugs, agents and / or compounds, may be locally or regionally delivered. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
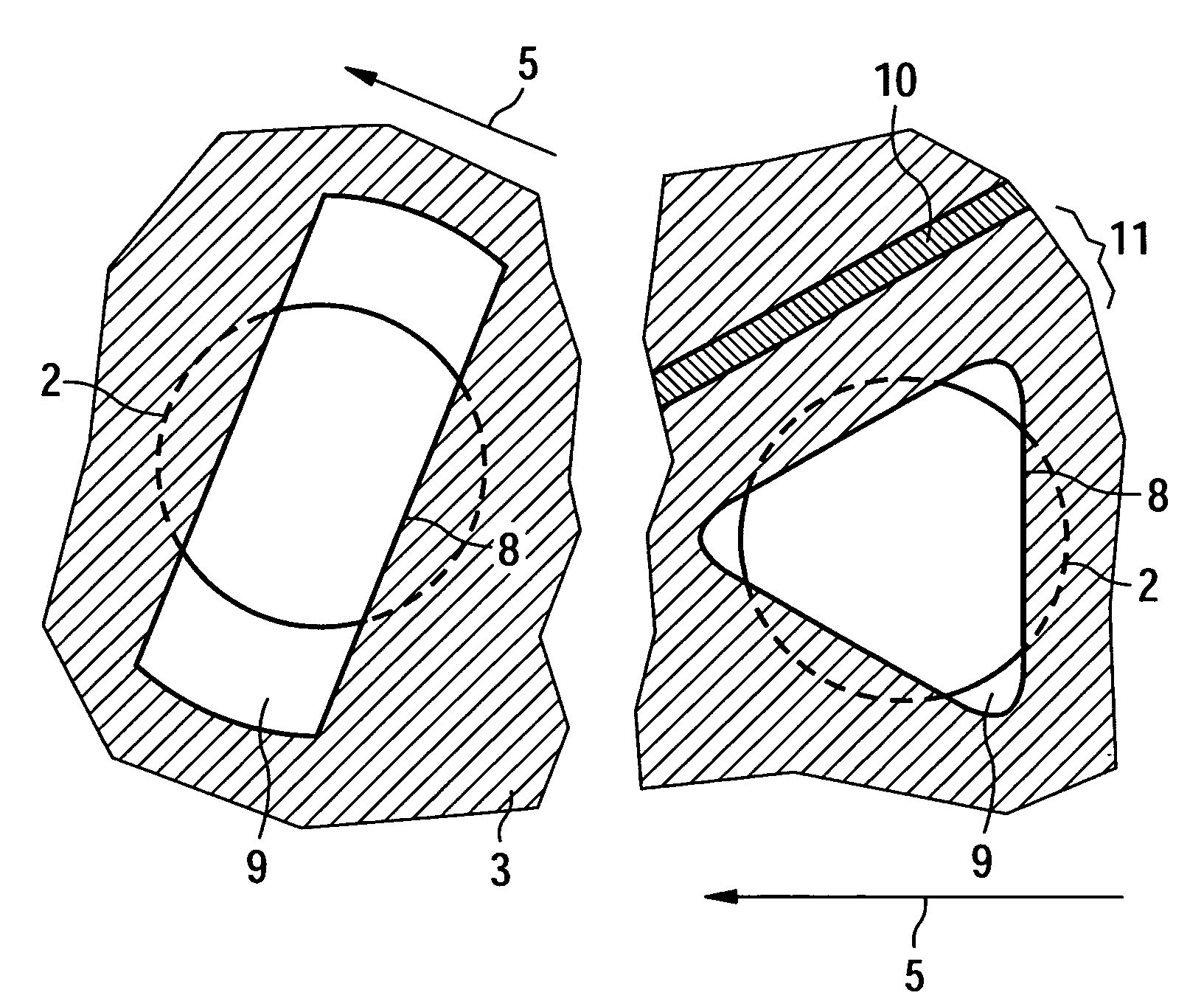
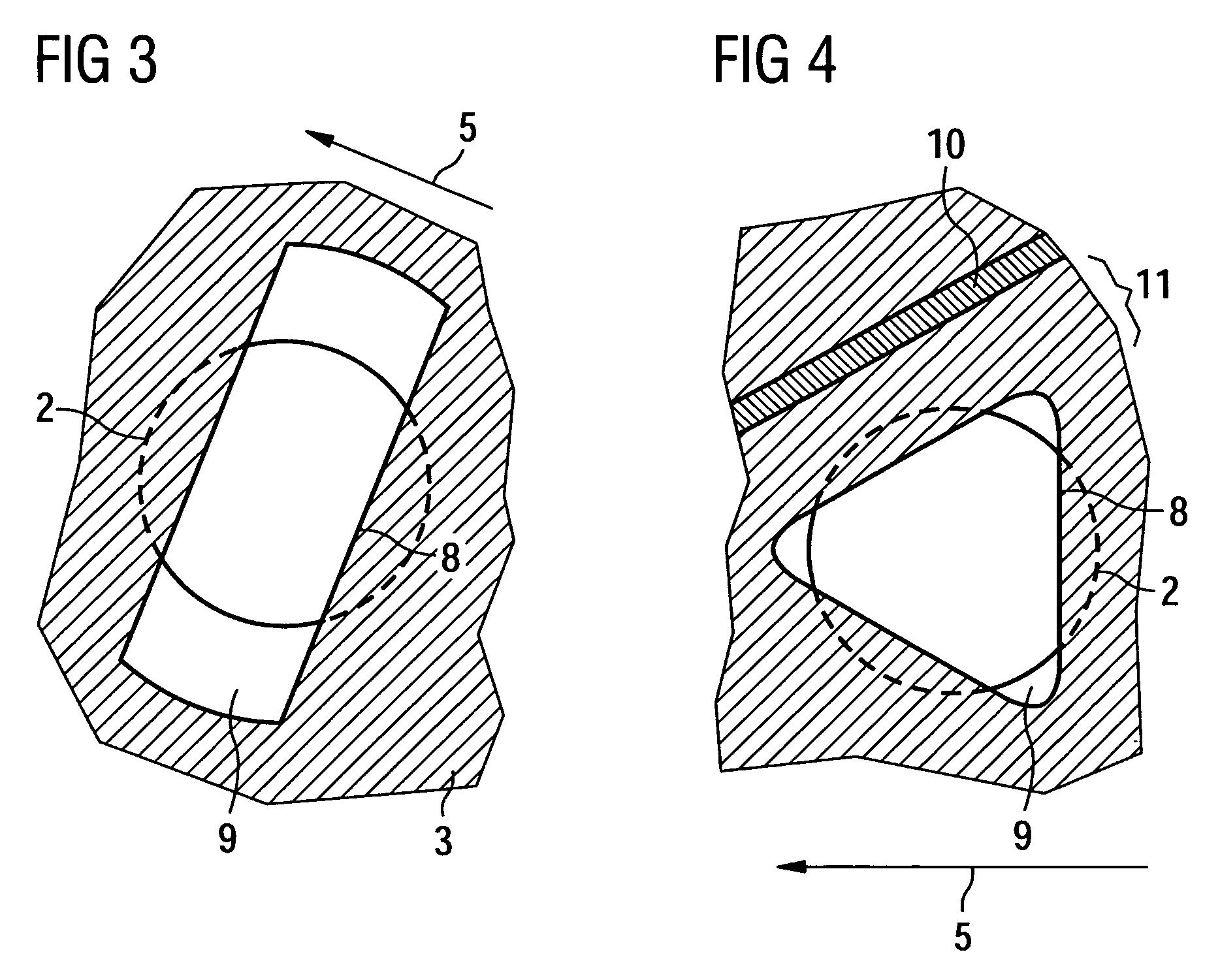
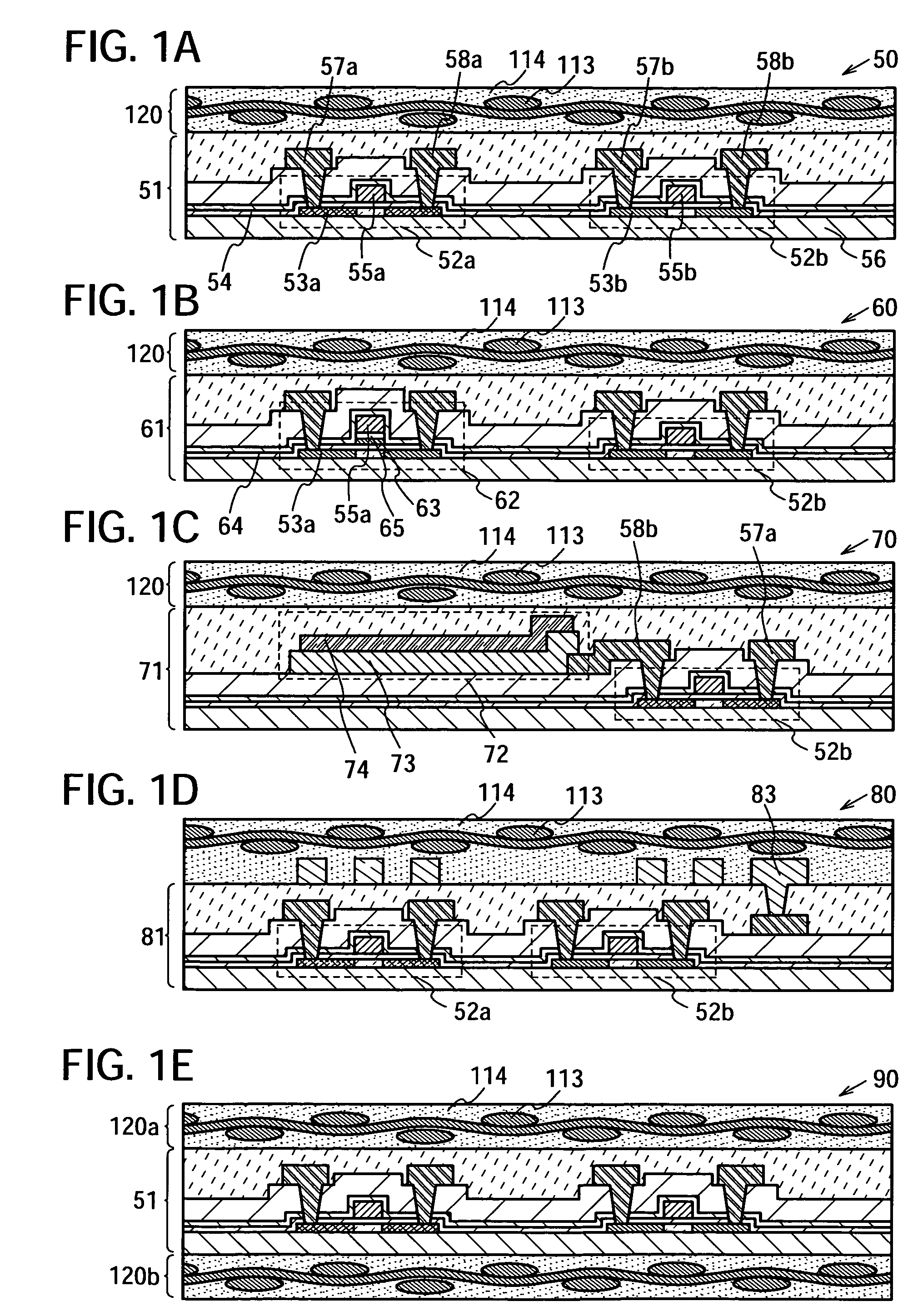
Semiconductor device and manufacturing method thereof
InactiveUS20080224941A1Avoid partialAvoid destructionSemiconductor/solid-state device detailsSolid-state devicesFiberInorganic compound
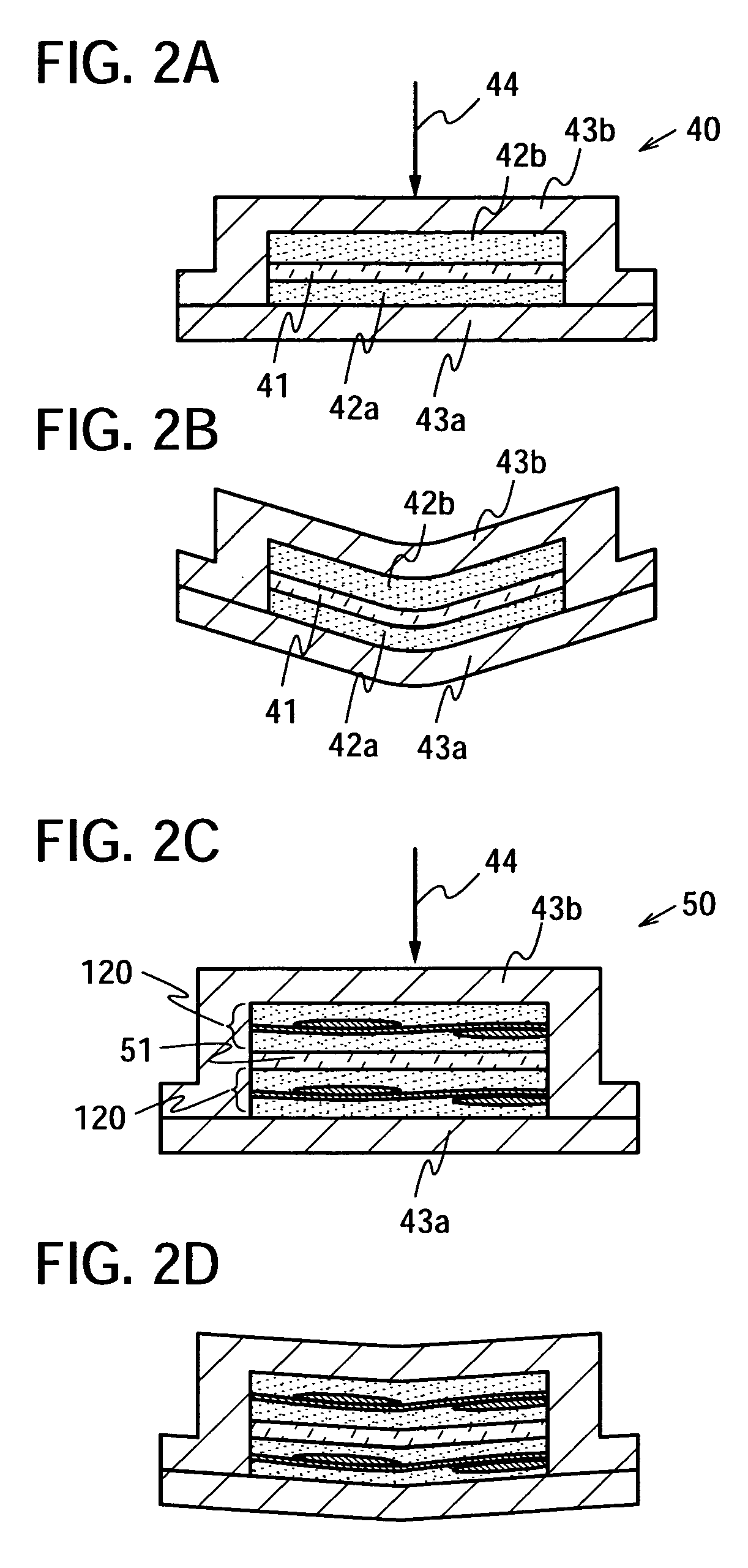
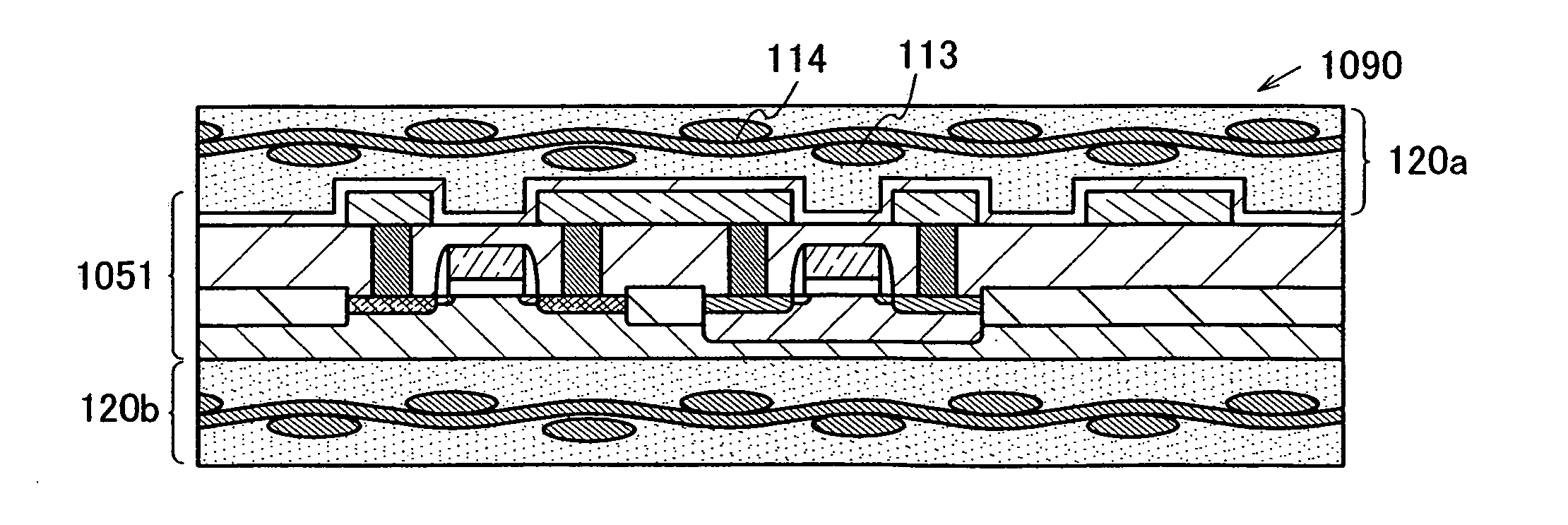
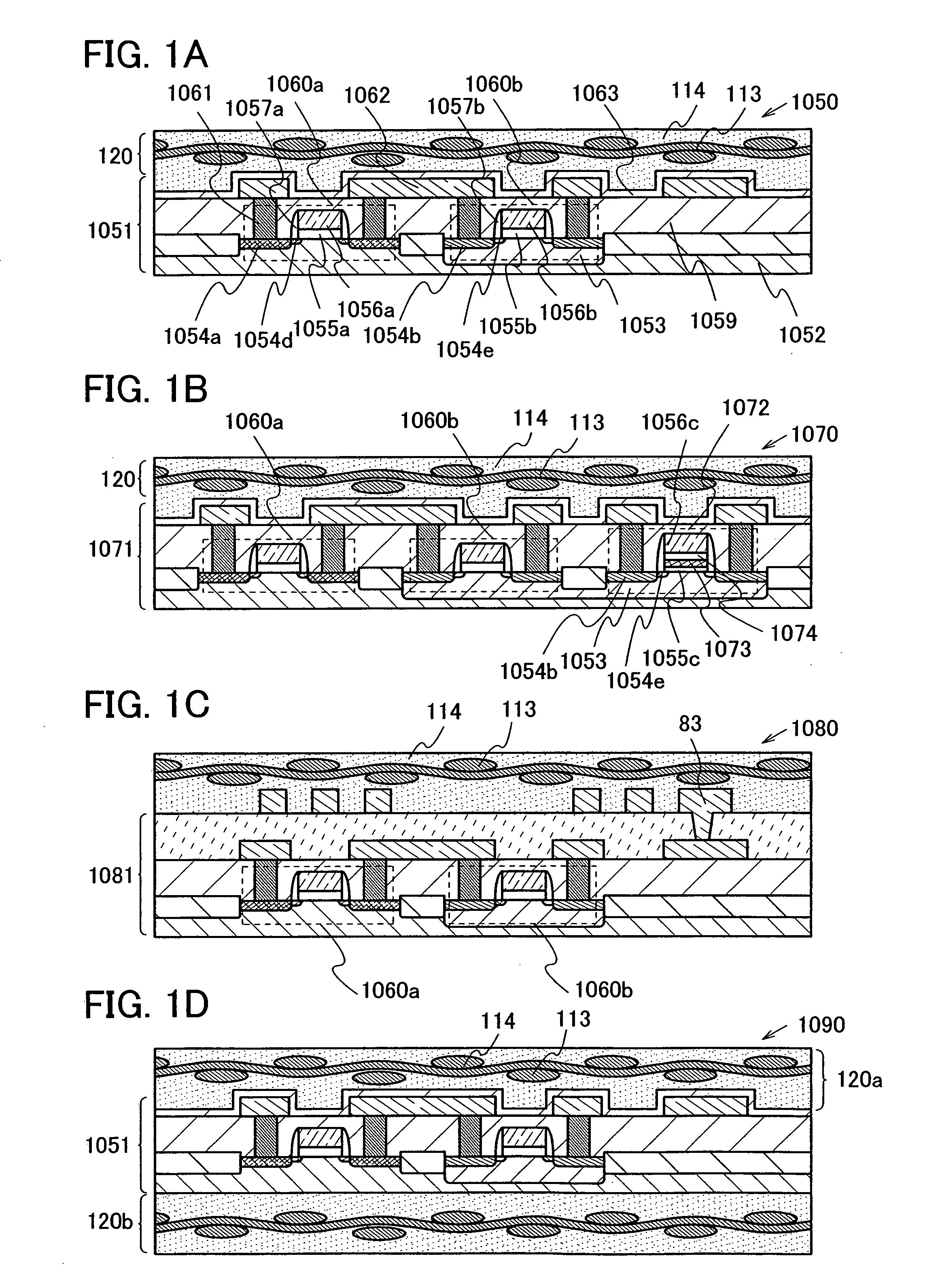
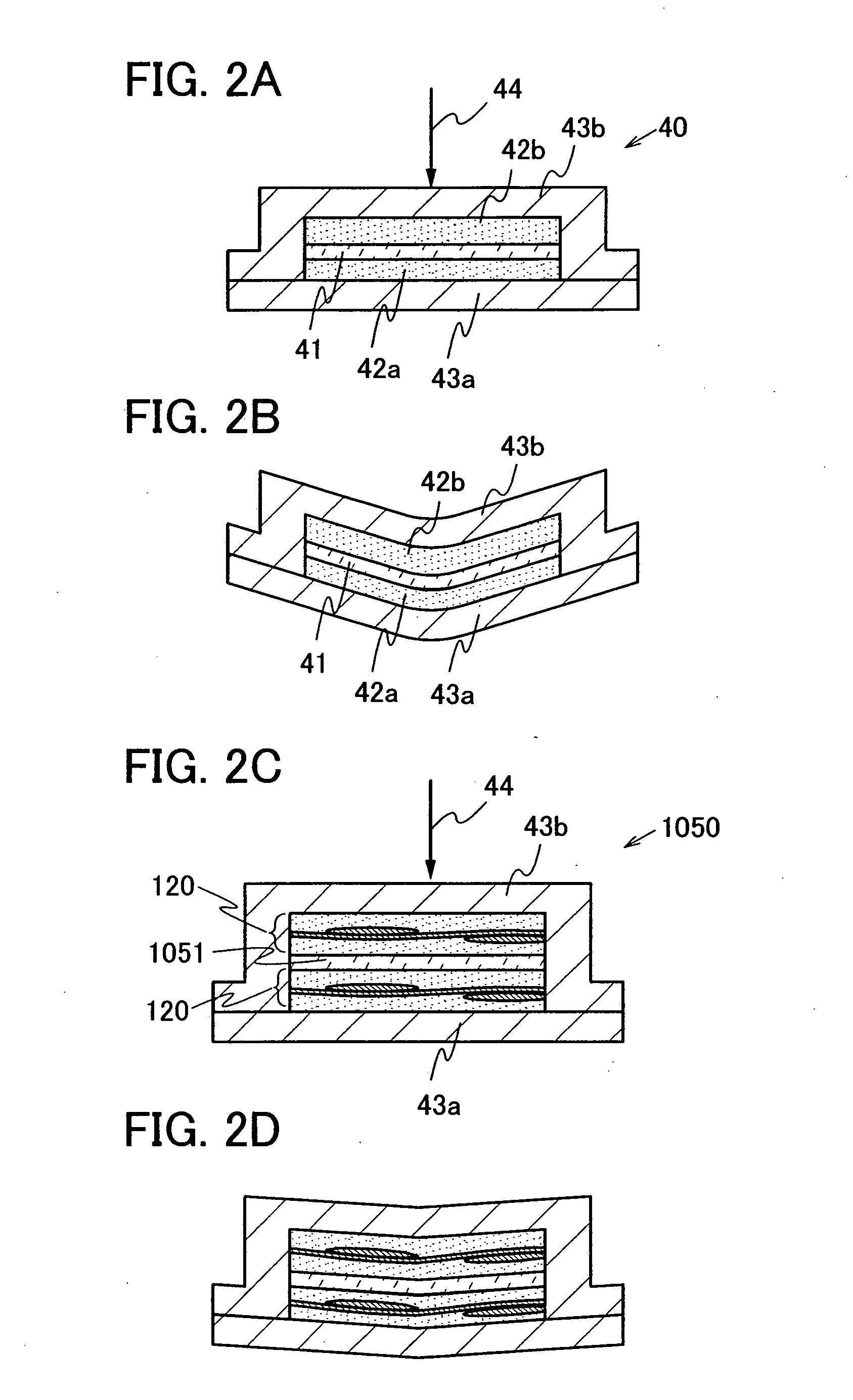
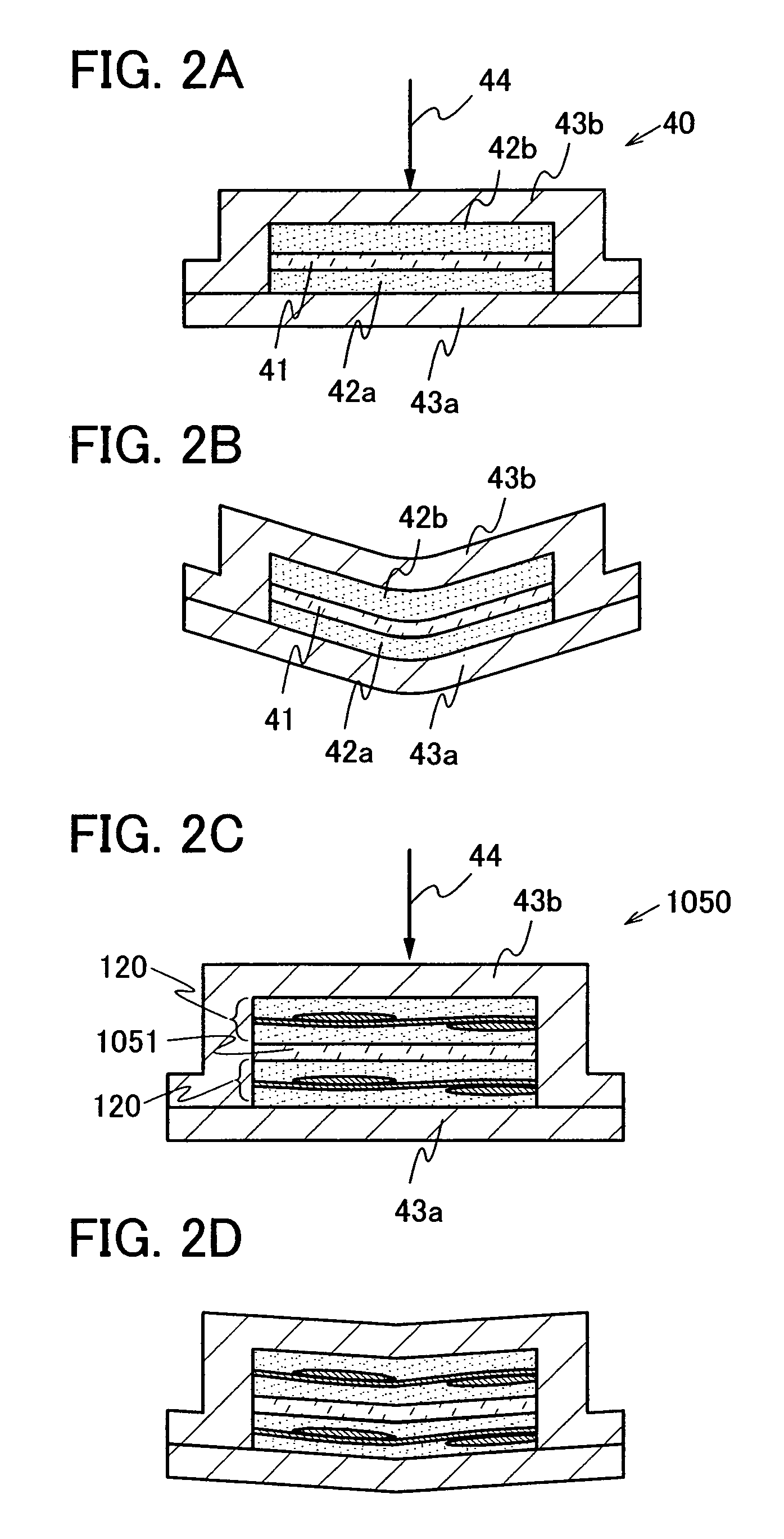
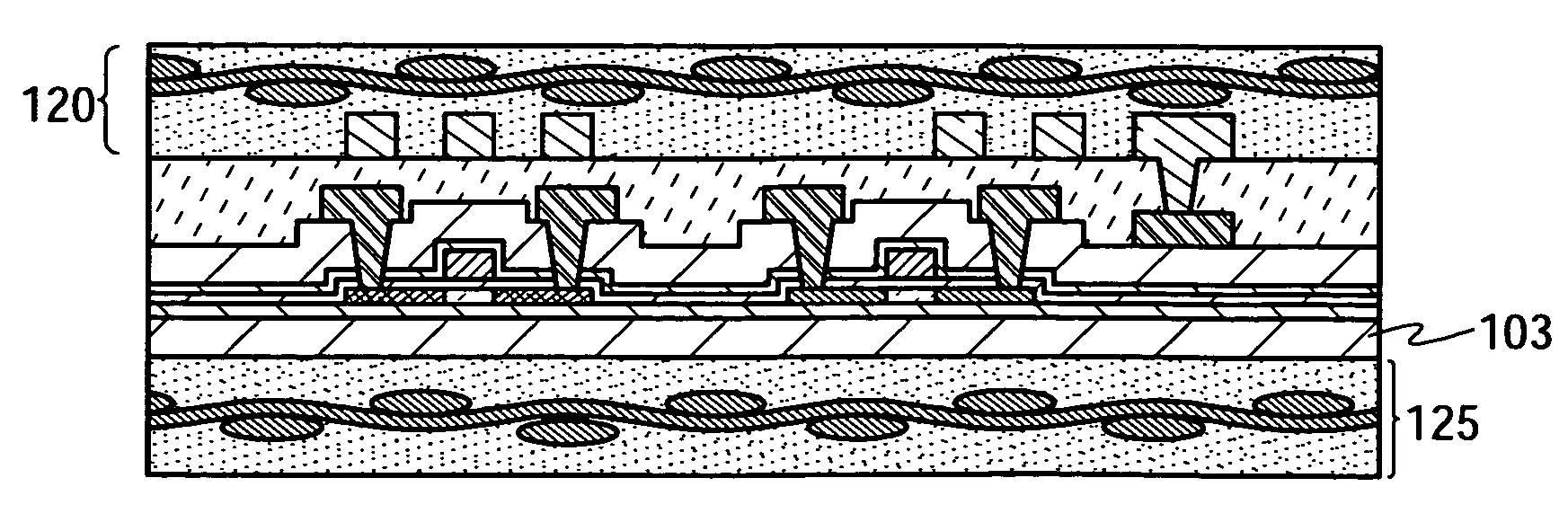
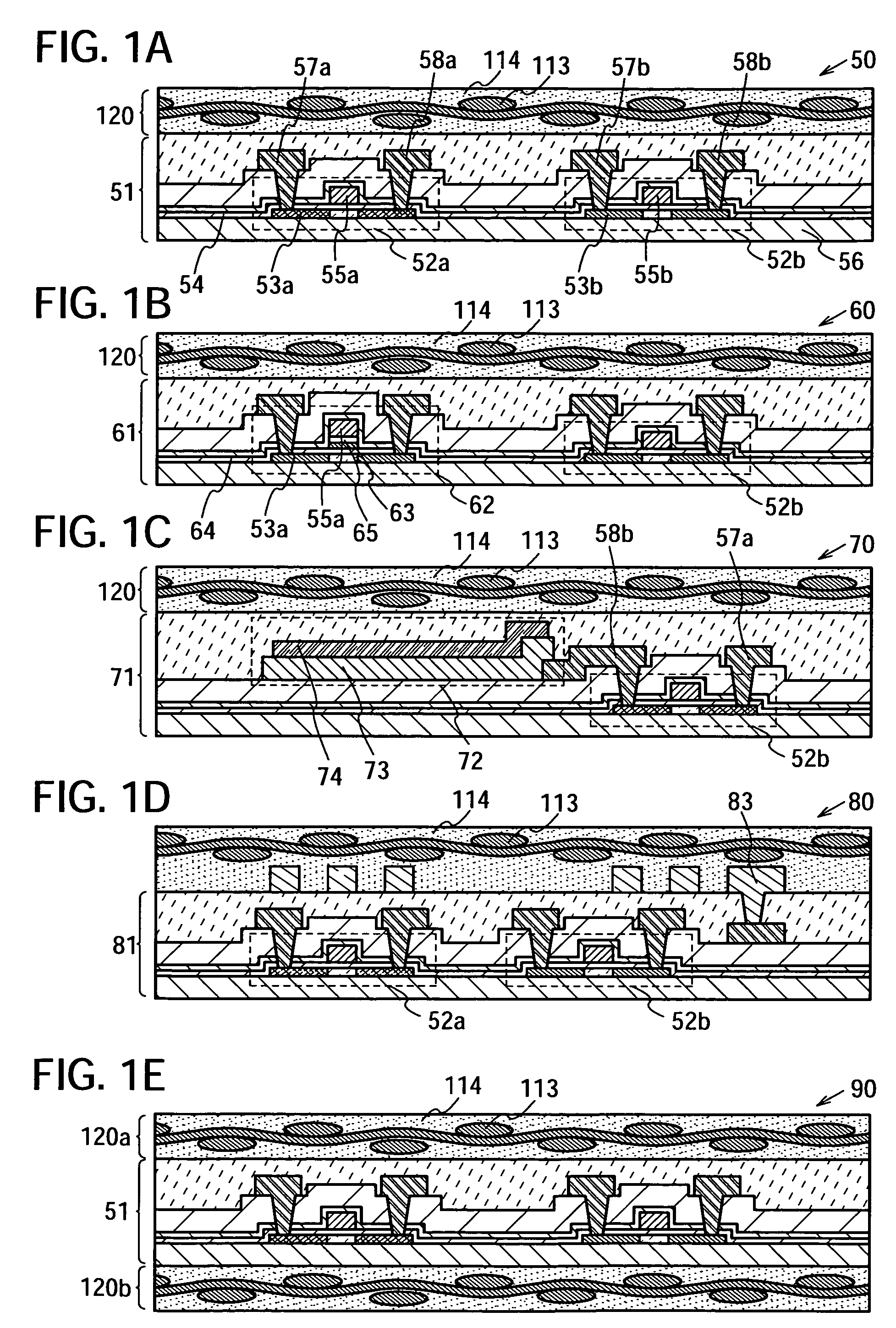
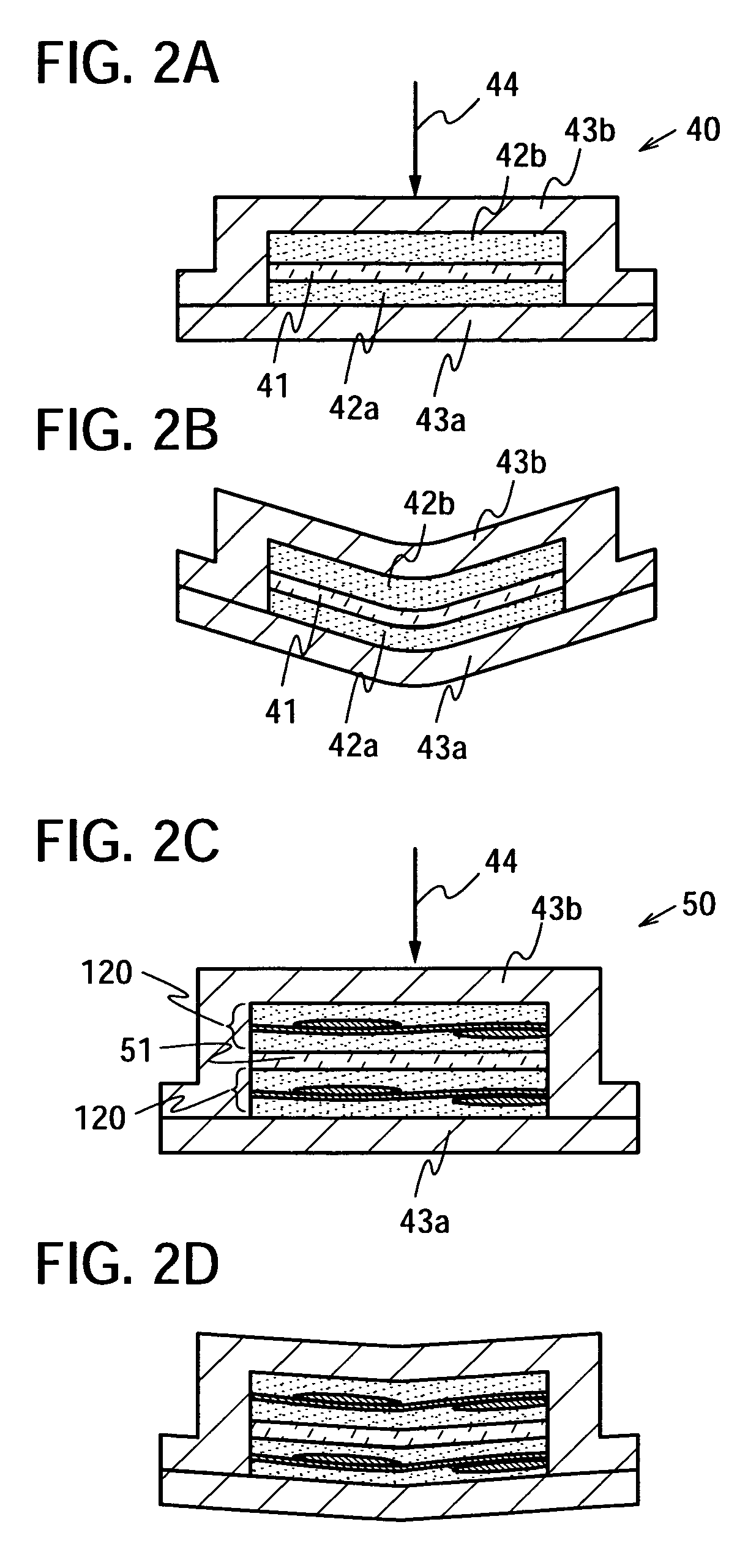
The present invention provides a semiconductor device which is not easily damaged by external local pressure. The present invention further provides a method for manufacturing a highly-reliable semiconductor device, which is not destructed by external local pressure, with a high yield. A structure body, in which high-strength fiber of an organic compound or an inorganic compound is impregnated with an organic resin, is provided over an element layer having a semiconductor element formed using a non-single crystal semiconductor layer, and heating and pressure bonding are performed, whereby a semiconductor device is manufactured, to which the element layer and the structure body in which the high-strength fiber of an organic compound or an inorganic compound is impregnated with the organic resin are firmly fixed together.
Owner:SEMICON ENERGY LAB CO LTD
Semiconductor device and manufacturing method thereof
InactiveUS20080224940A1Avoid partialAvoid destructionSemiconductor/solid-state device detailsSolid-state devicesFiberInorganic compound
The present invention provides a semiconductor device which is not easily damaged by external local pressure. The present invention further provides a manufacturing method of a highly-reliable semiconductor device, which is not destroyed by external local pressure, with a high yield. A structure body, in which high-strength fiber of an organic compound or an inorganic compound is impregnated with an organic resin, is provided over an element substrate having a semiconductor element formed using a single crystal semiconductor region, and heating and pressure bonding are performed, whereby a semiconductor device is manufactured, to which the element substrate and the structure body in which the high-strength fiber of an organic compound or an inorganic compound is impregnated with the organic resin are fixed together.
Owner:SEMICON ENERGY LAB CO LTD
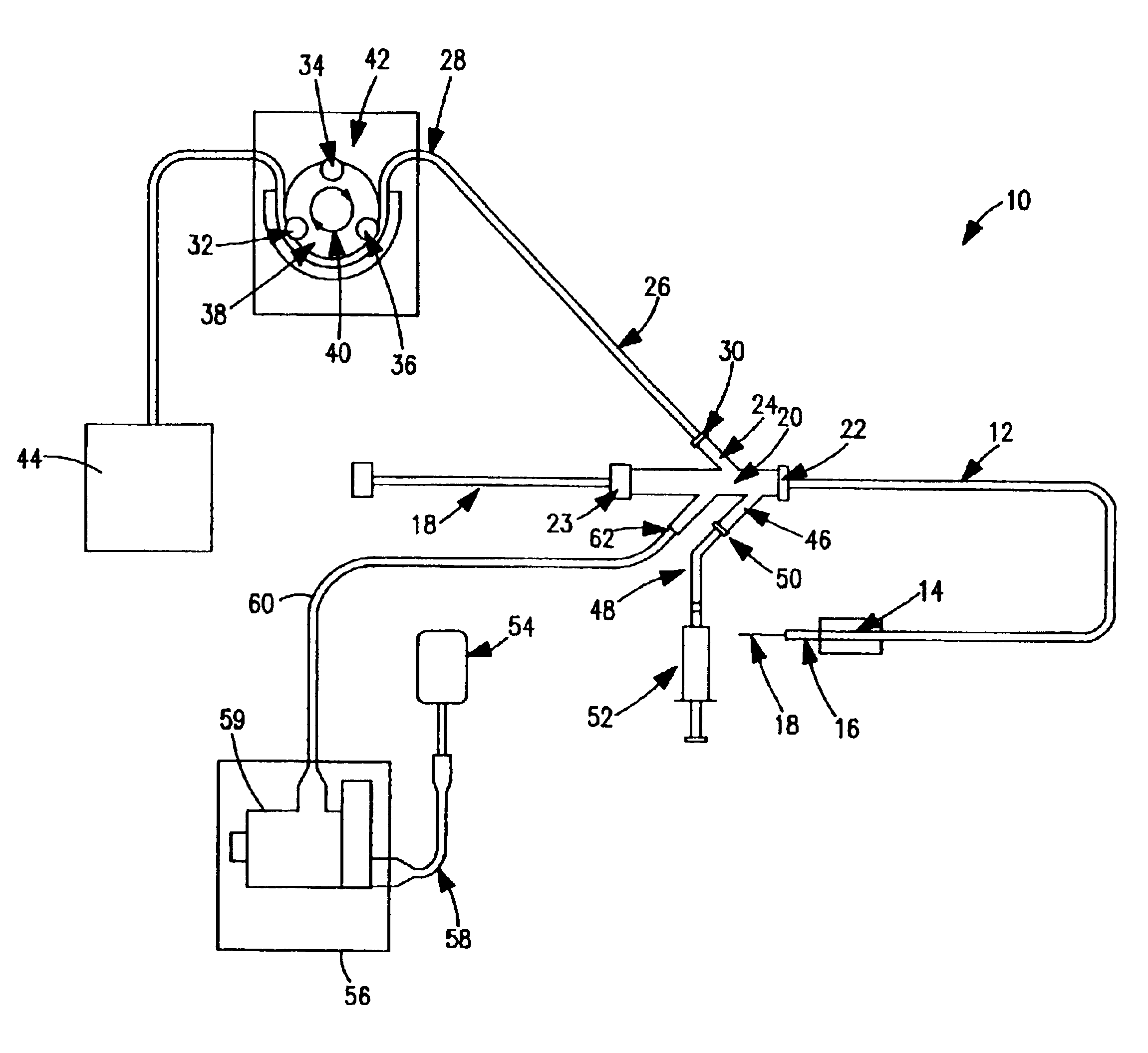
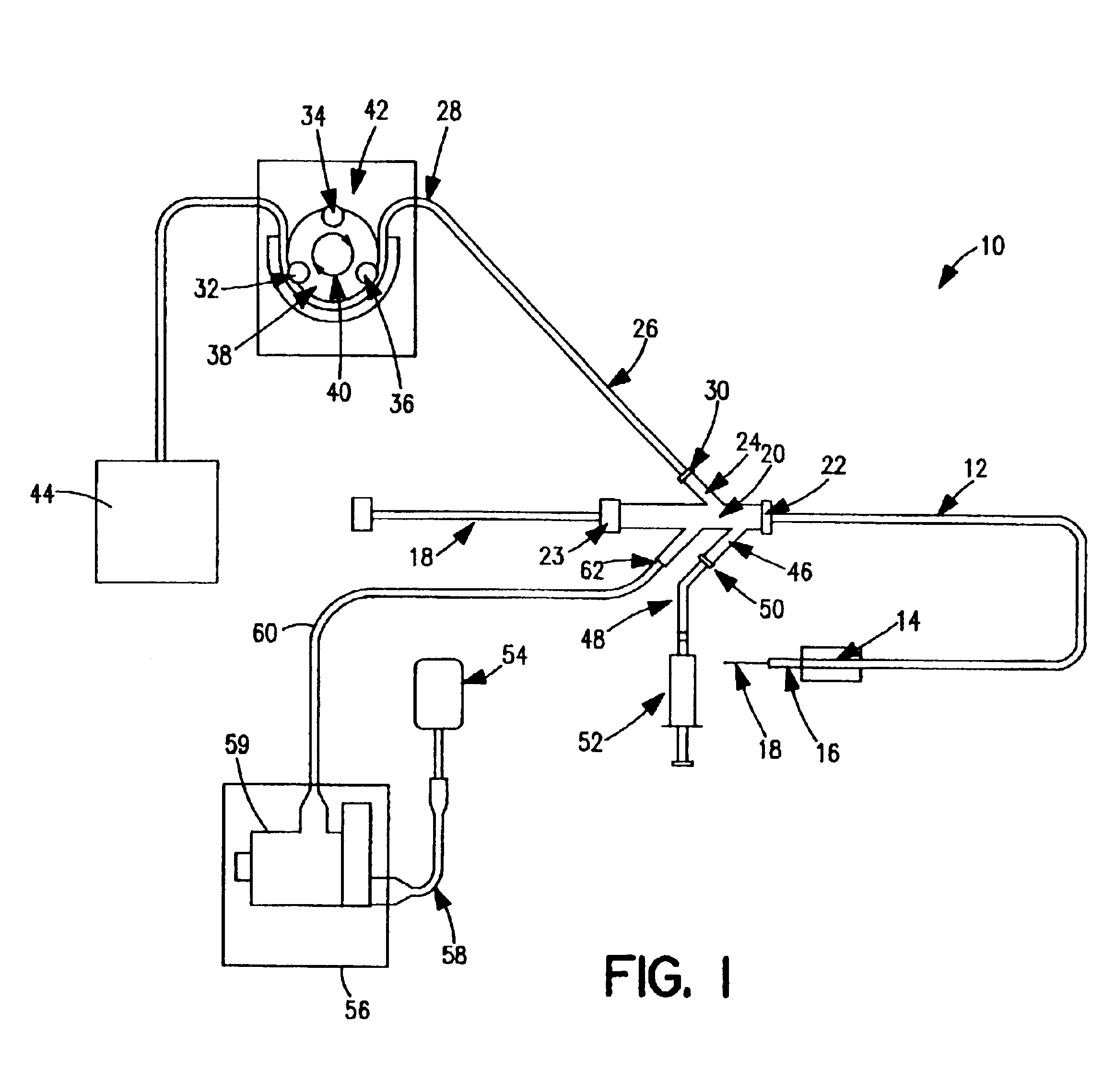
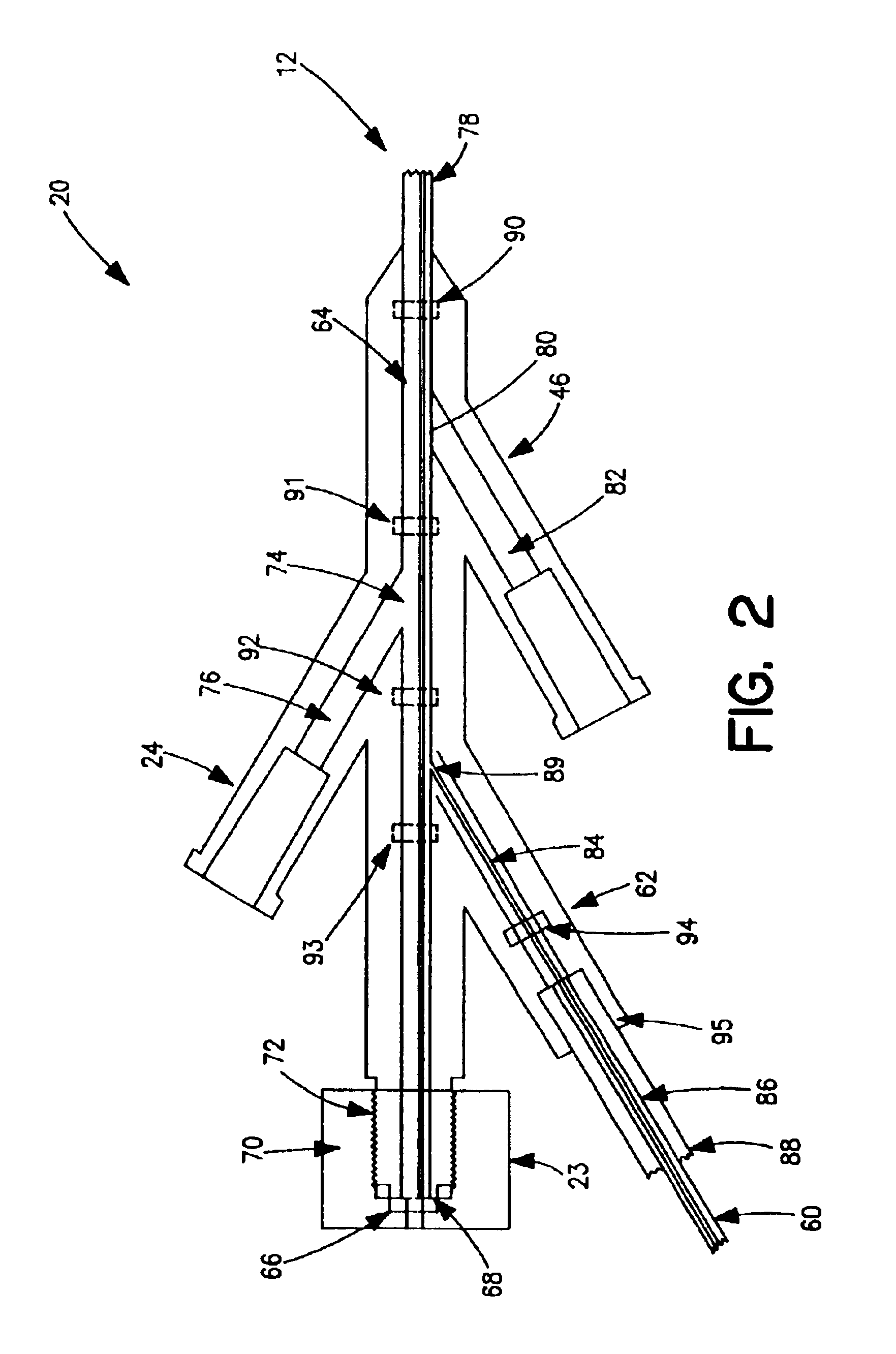
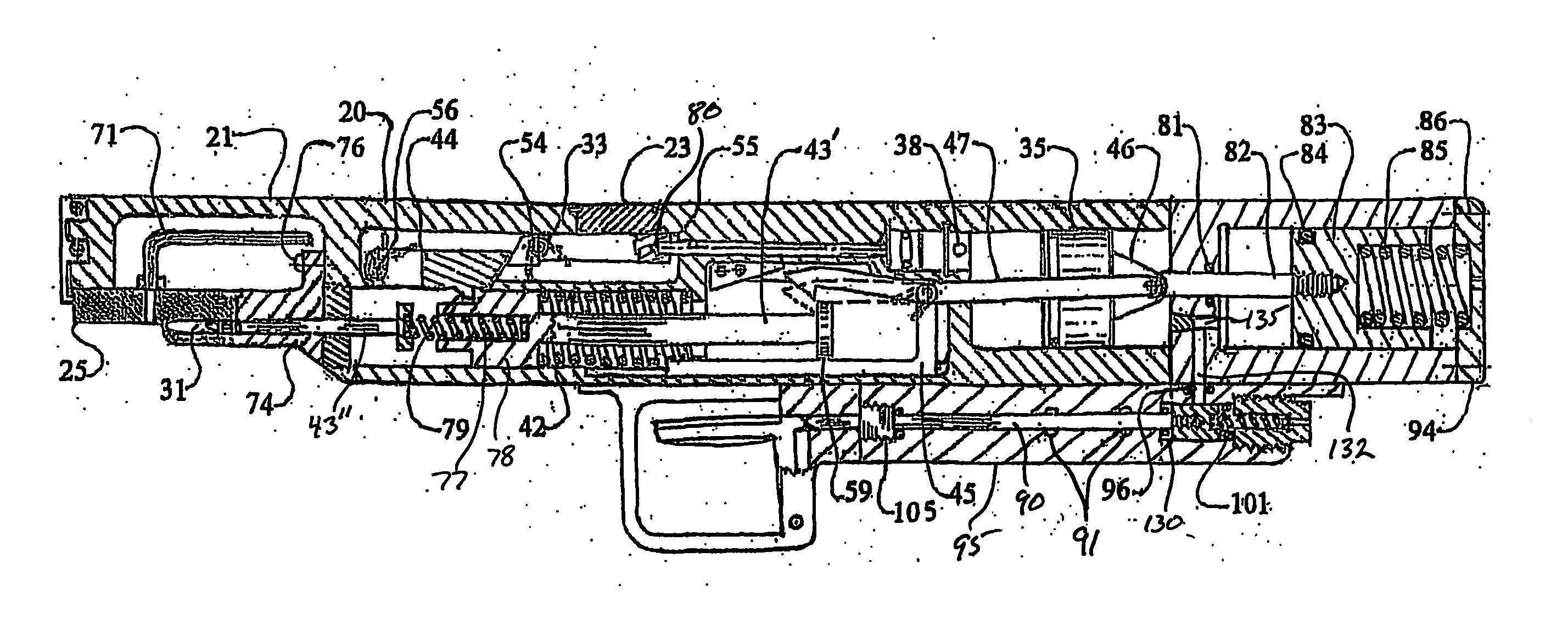
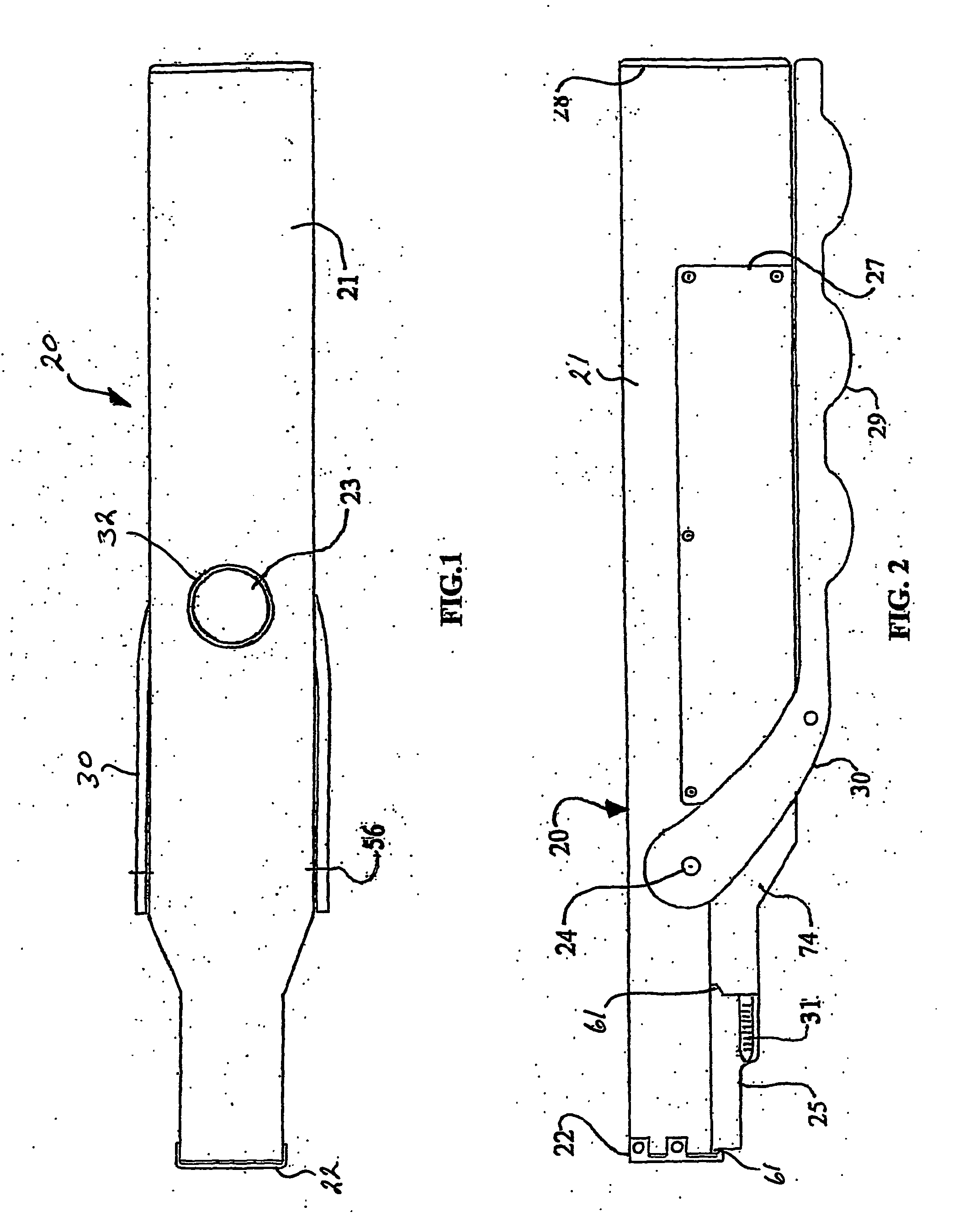
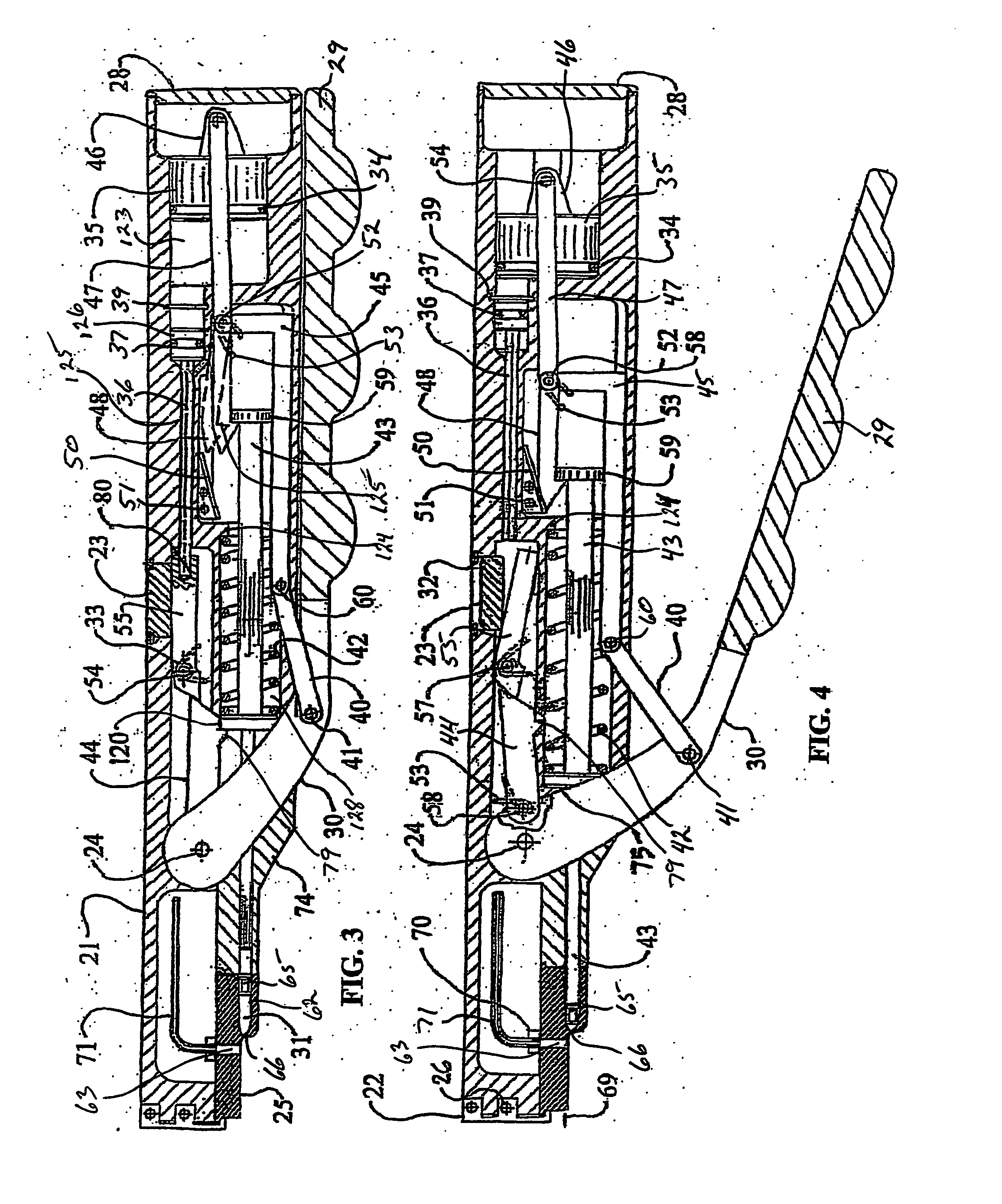
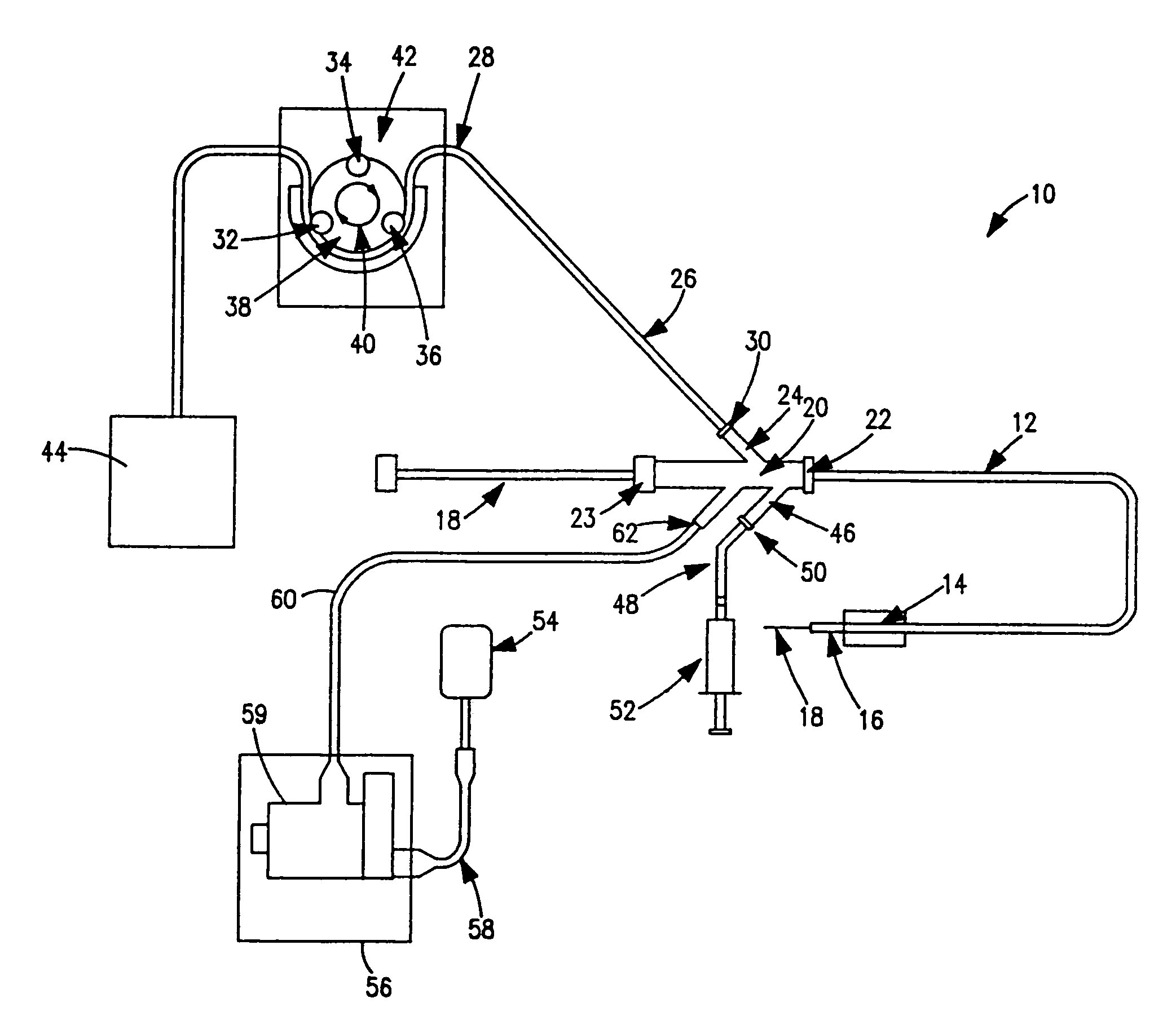
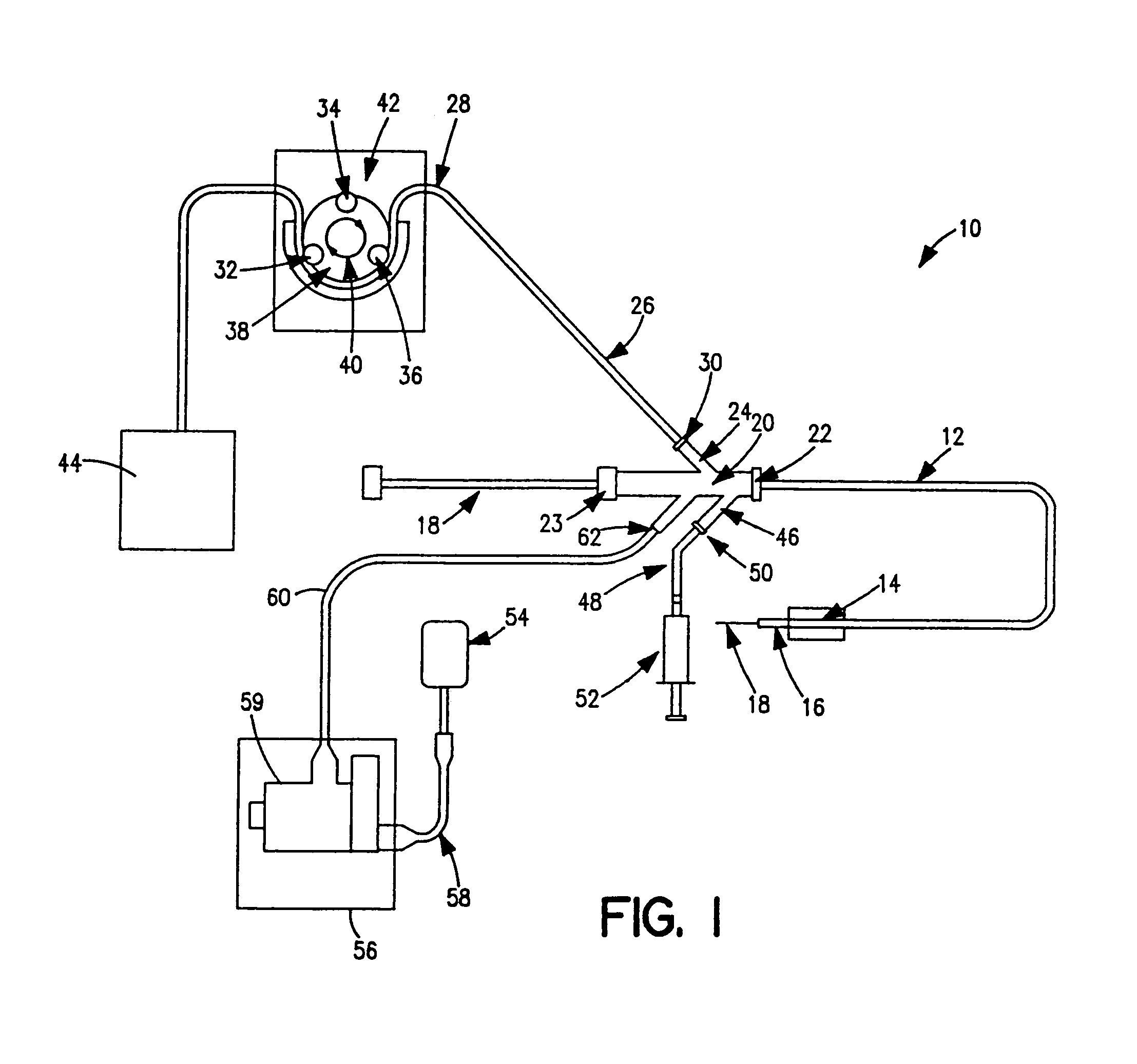
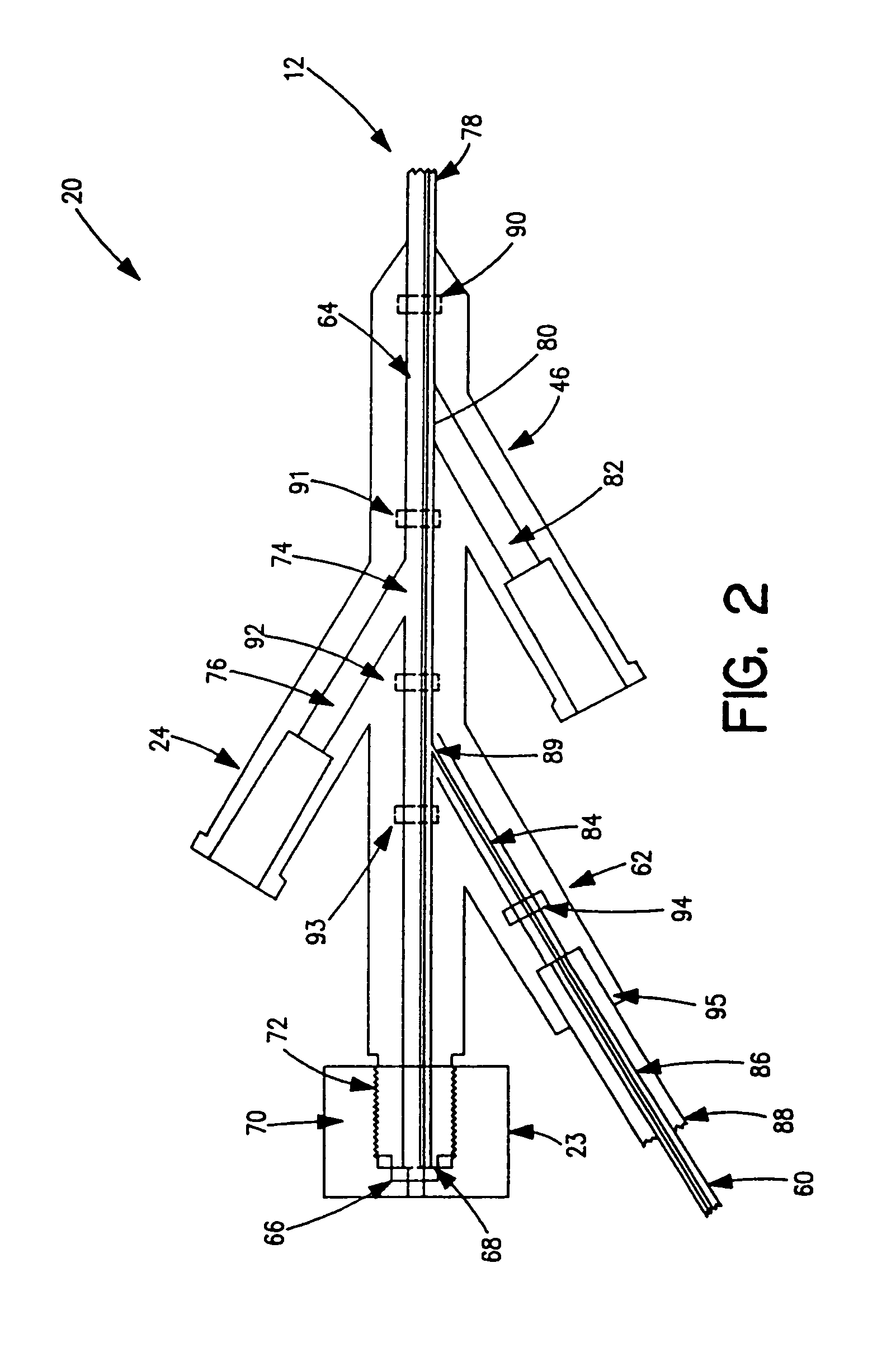
Thrombectomy and tissue removal method
InactiveUS6926726B2Increase speedReduce local pressureCannulasSurgical instrument detailsThrombusHigh pressure
A method for removing thrombus or other material from a natural or synthetic body vessel or cavity without the need for direct surgical access. The method includes providing a device supplying high pressure fluid to at least one distal orifice, causing high pressure fluid to emanate from the orifice creating at least one fluid jet, and using the fluid jet to break up material in the vessel. The method further includes directing at least one fluid jet at the opening of an exhaust lumen or target incorporated into the device and using the jet to provide a localized negative pressure which entrains material into the jet for break-up. The method optionally includes using the jet to provide stagnation pressure which drives material along the exhaust lumen. The method optionally includes metering the exhaust to match the fluid input or to be greater or less than the input. A positive displacement pump operating at steady or pulsatile flow provides the high pressure saline to the distal end of the catheter.
Owner:MEDRAD INC.
Needle free hypodermic injector and ampule for intradermal, subcutaneous and intramuscular injection
InactiveUS20070167907A1Reduce local pressureEliminate leaksJet injection syringesAutomatic syringesNeedle freeSuction force
A needle free hypodermic injector comprising a hand manipulatable elongate housing, an impact impulse injection mechanism within the housing, a suction generating means within the housing and cooperable with the impact impulse injection mechanism, a safety interlock mechanism, at least one medicament containing ampule cooperable with the impact impulse injection mechanism and the suction generating means and having a jet orifice through which medicament is injectable through a skin surface in response to an impulse placed on the medicament by the impact impulse injection mechanism, and means to receive and hold the ampule on the injector in registration with the impact impulse injection mechanism and in communication with the suction generating means; whereby the injector is adapted to expel the medicament from the ampule in a jet stream of sufficient velocity to penetrate skin tissue held against the orifice by the suction generating means and to deposit the medicament intradermally, subcutaneously or intramuscularly based on the position and angle of the jet orifice.
Owner:DESLIERRES JOHN +2
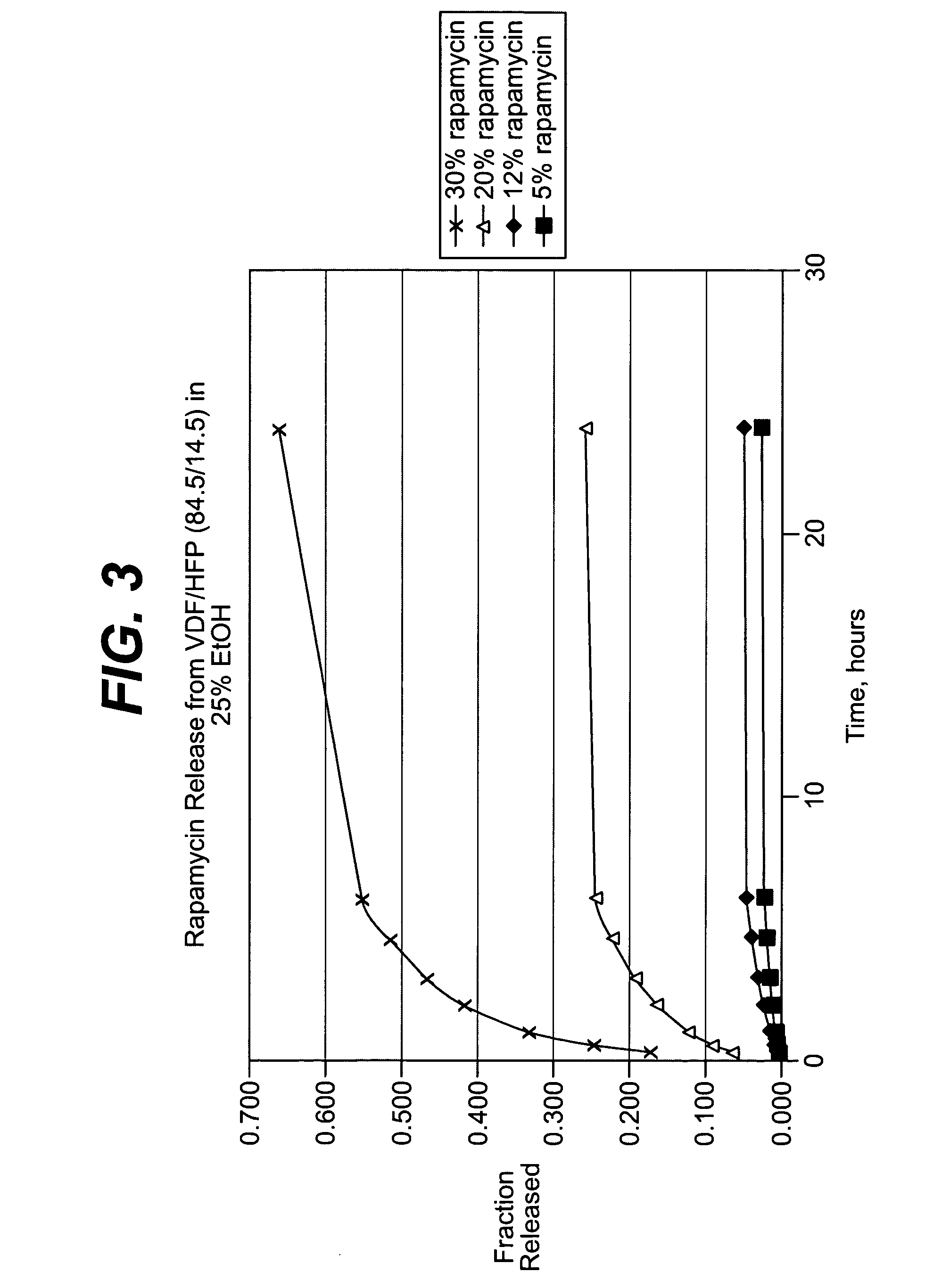
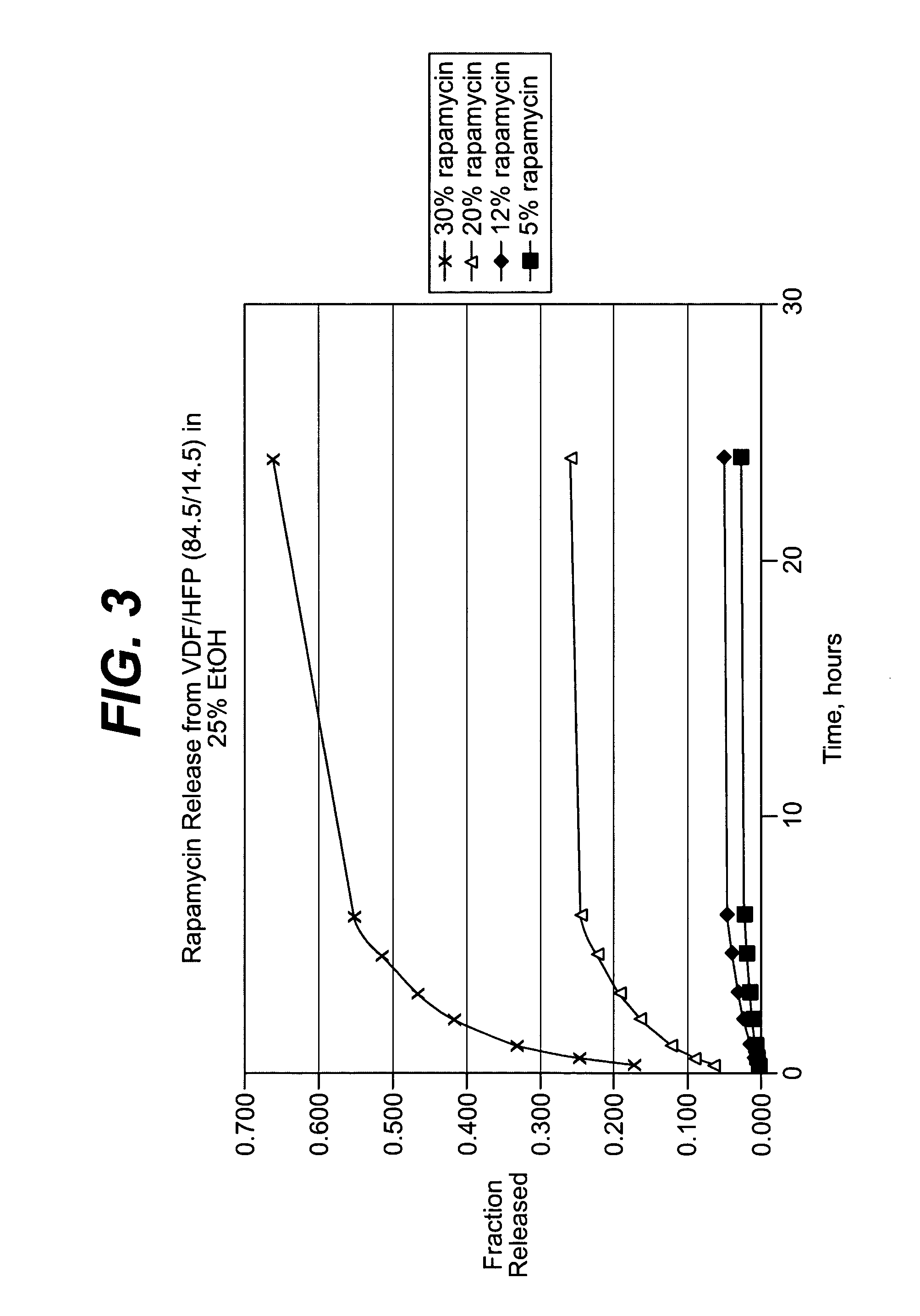
Local vascular delivery of cladribine in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS20050182485A1Minimize potential risk of damageReduce frictionSuture equipmentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
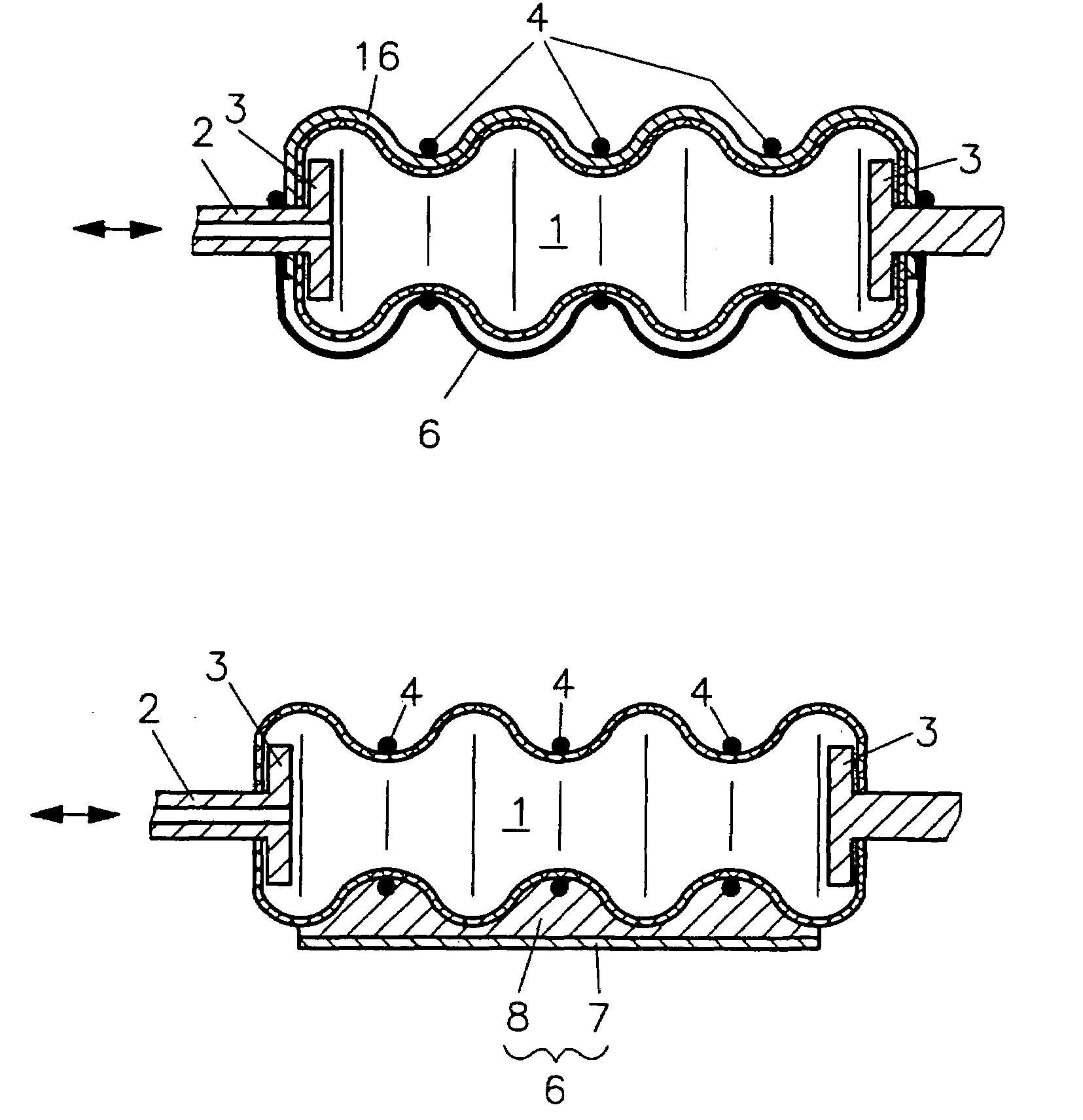
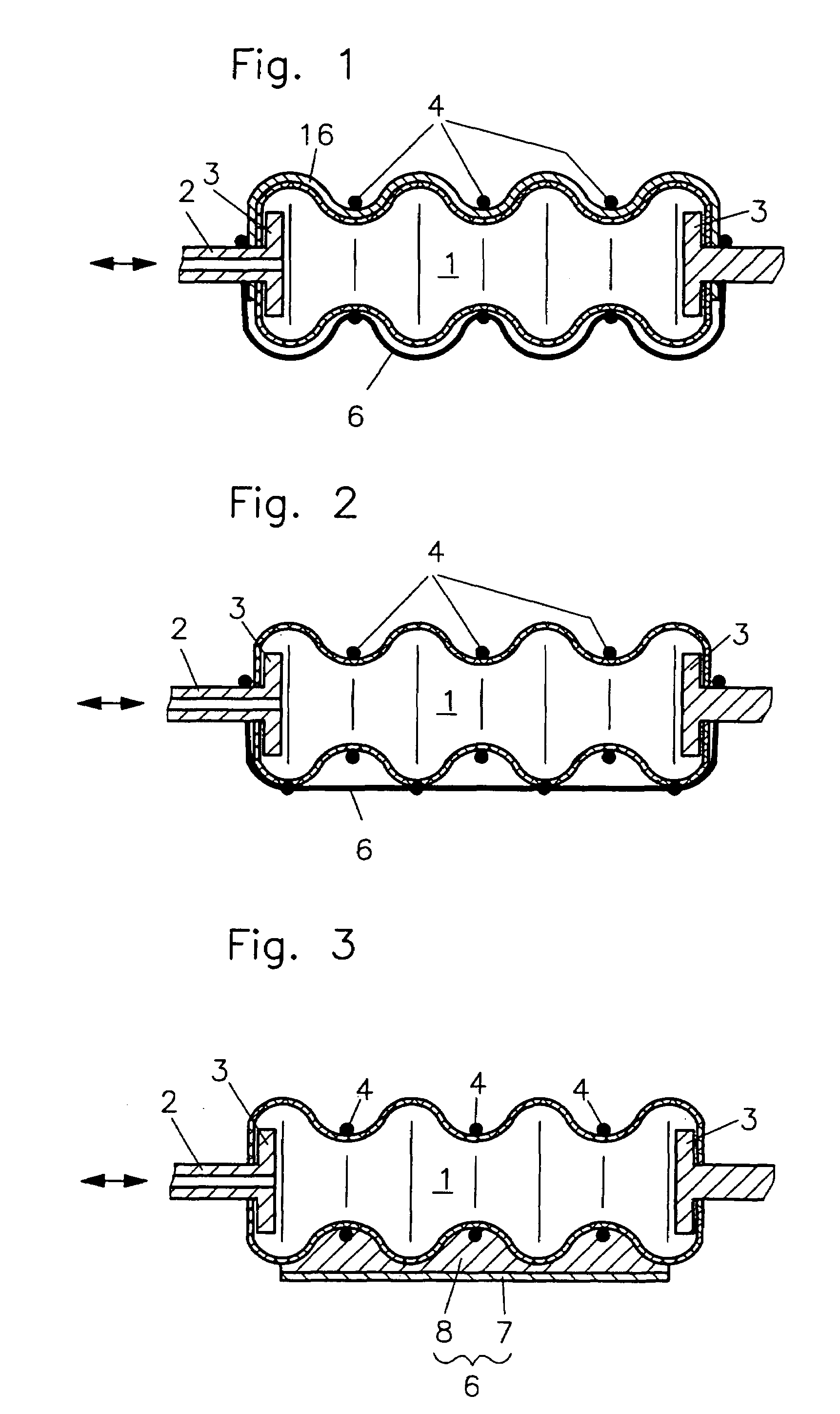
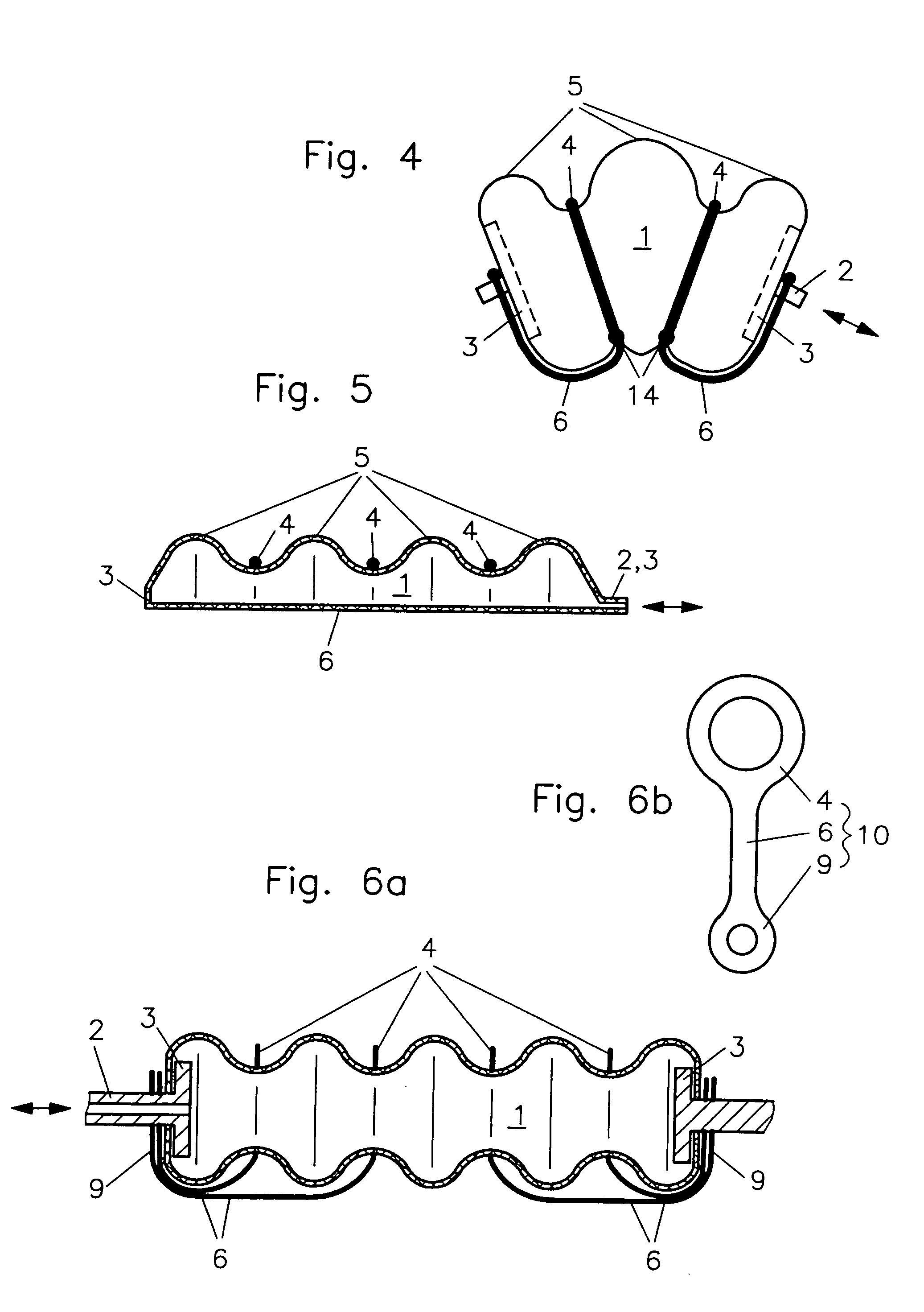
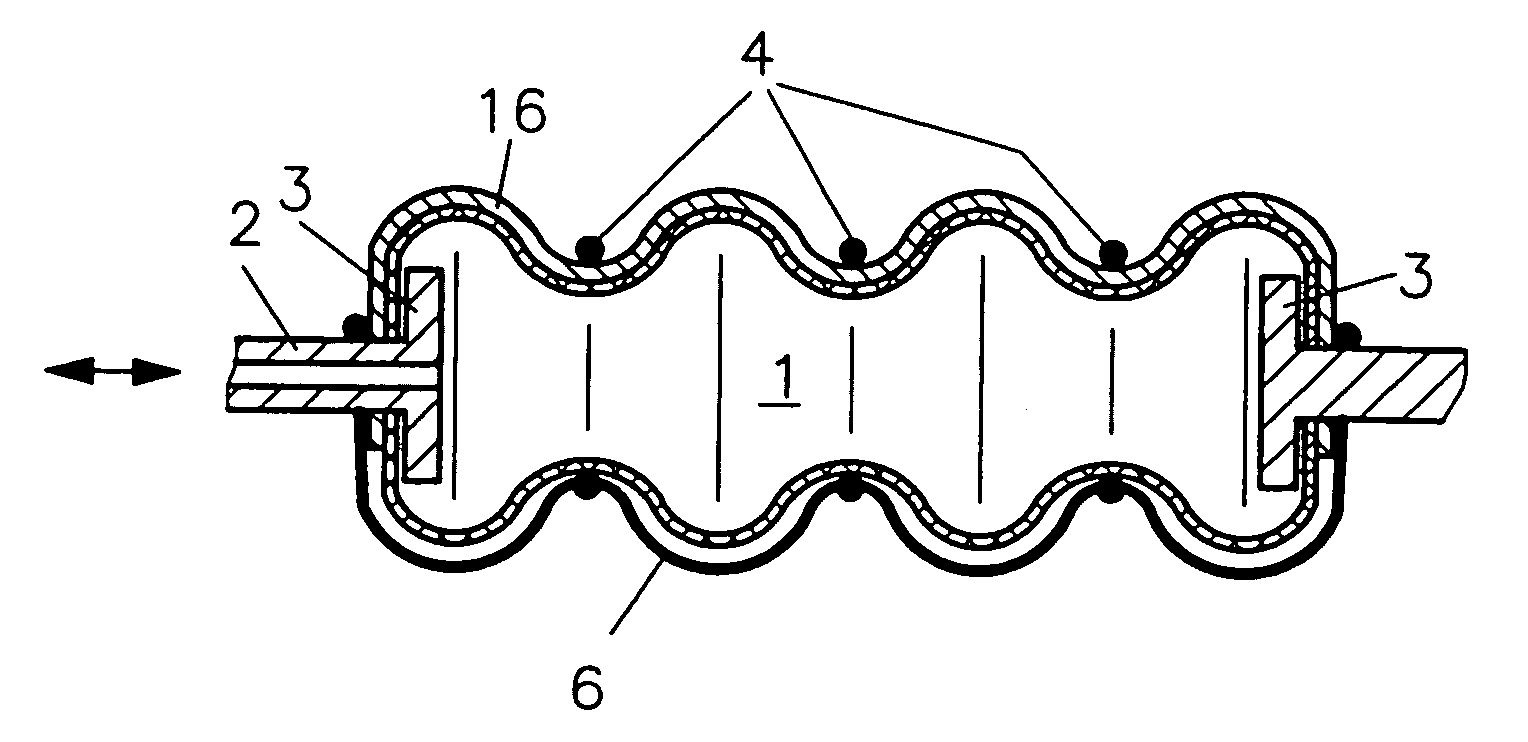
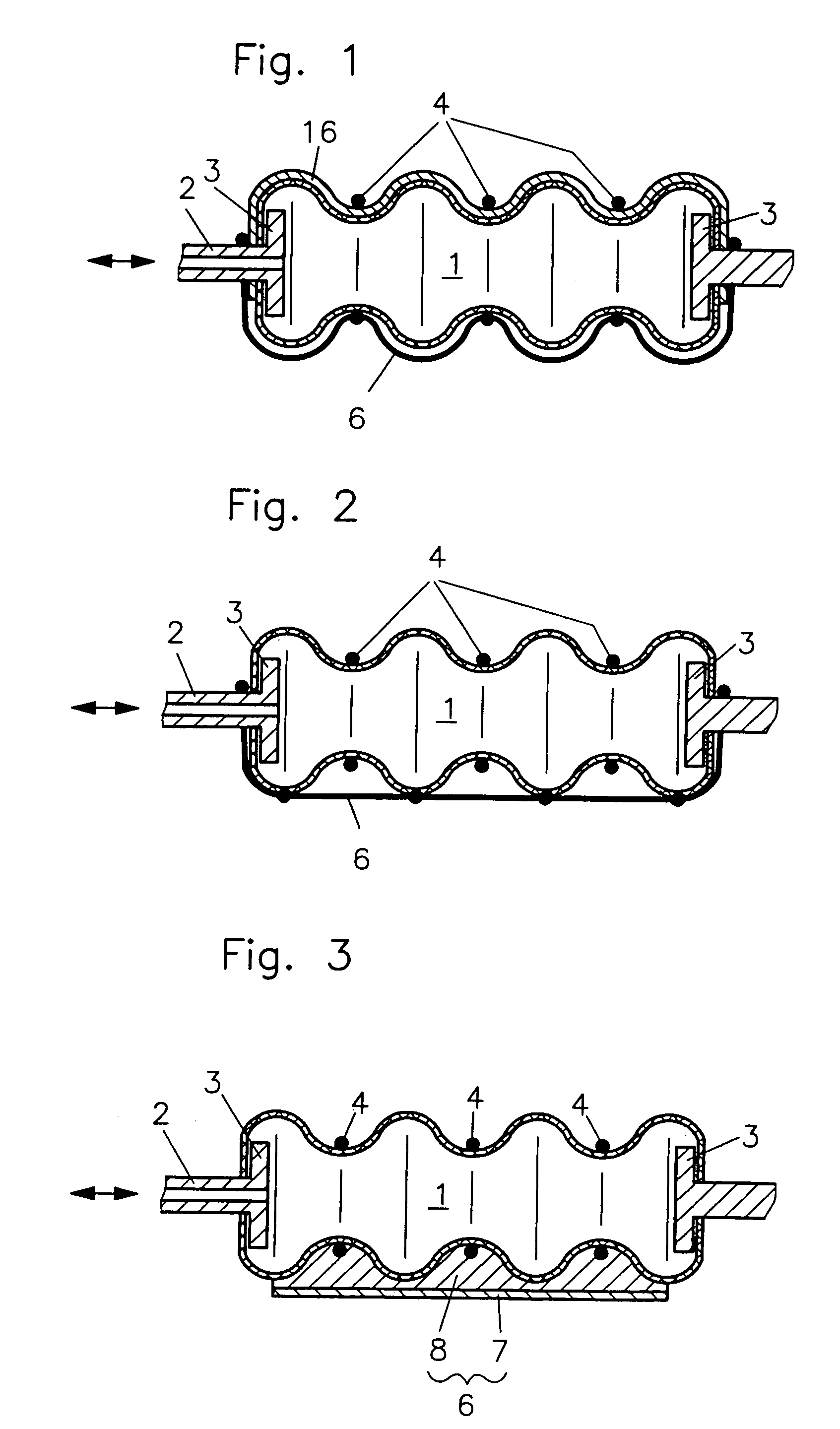
Fluidic device
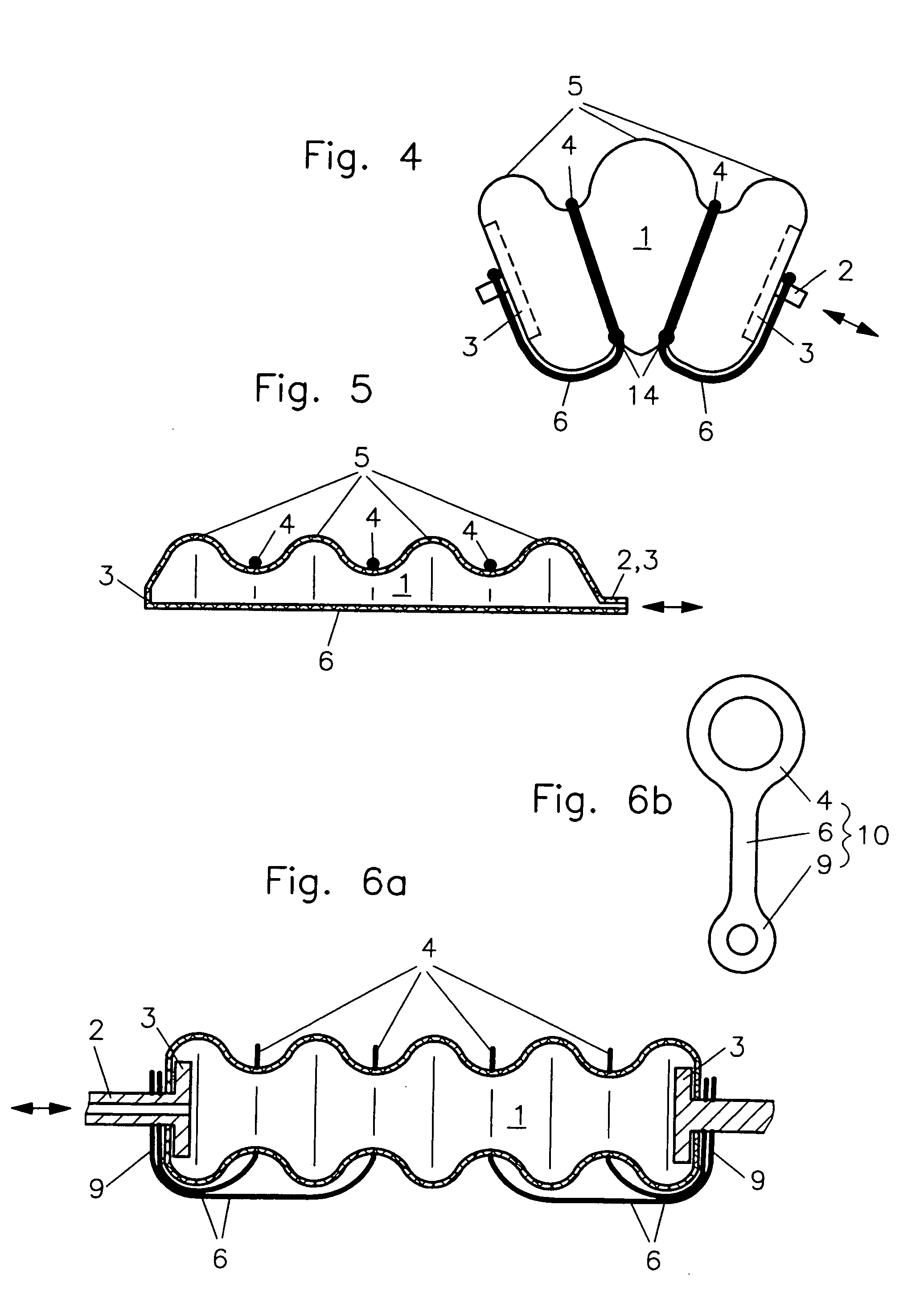
InactiveUS7086322B2Easy to controlAccurate doseFlexible wall reciprocating enginesFluid-pressure actuatorsEngineeringRing element
In a fluidic drive for disposition between two components which are movable relative to each other comprising a hollow body with a duct for supplying fluid to, and discharging it from, the hollow body wherein the hollow body consists at least partially of a bellows structure, non-resilient ring elements extend around the hollow body in each of its pleats and a connecting structure extends at least at one side of the hollow body and interconnects the non-resilient ring elements.
Owner:GSI HELMHOLTZZENT FUR SCHWERIONENFORSCHUNG
Thrombectomy and tissue removal method
InactiveUS6984239B1Reduce local pressureIncrease speedMedical devicesFluid jet surgical cuttersThrombusHigh pressure
A device for removing a thrombus or other tissue deposit from the cardiovascular system, natural or synthetic tubule or cavity found in the human body of a patient without the need to surgically access the location of the thrombus or other tissue deposit via a cut-down or other surgical procedure. A flexible metal or high pressure plastic tube conveys an extremely high pressure stream of sterile saline or other physiologic solution to at least one jet at the distal end of the catheter. At least one jet is directed at the opening of a large exhaust lumen or other target. The jet(s) is responsible for providing a localized negative pressure which entrains tissue into the jet from break-up of the debris. This jet(s) can also provide stagnation pressure in the exhaust lumen which drives the tissue or thrombotic debris out of the exhaust lumen. Operation of the device with tip pressure greater than 500 psi provides this device with the entrainment and exhaust characteristics which contribute to its effectiveness. The rate of exhaust of tissue debris is metered to ensure minimal local impact on the vasculature at the site of the thrombus deposit. A fluid metering means, such as a roller pump, controls the rate of exhaust such that it is in balance with the saline input or can be adjusted to be greater or less than the input. A positive displacement pump operating at steady or pulsatile flow provides the high pressure saline to the tip of the catheter.
Owner:MEDRAD INC.
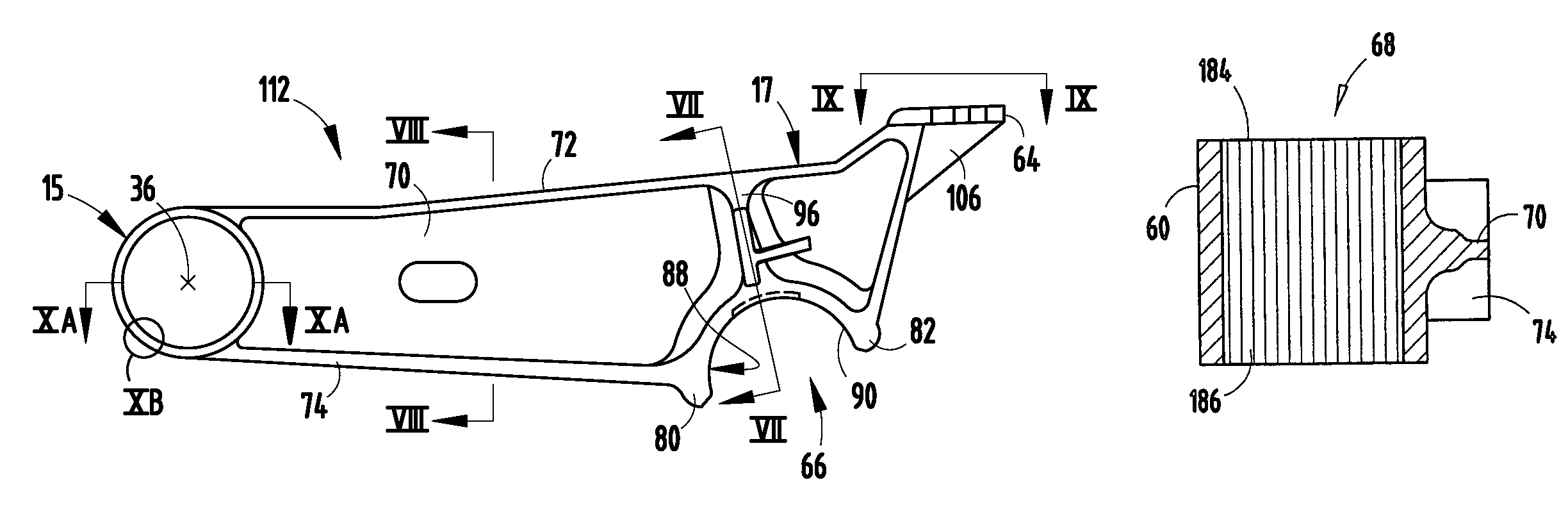
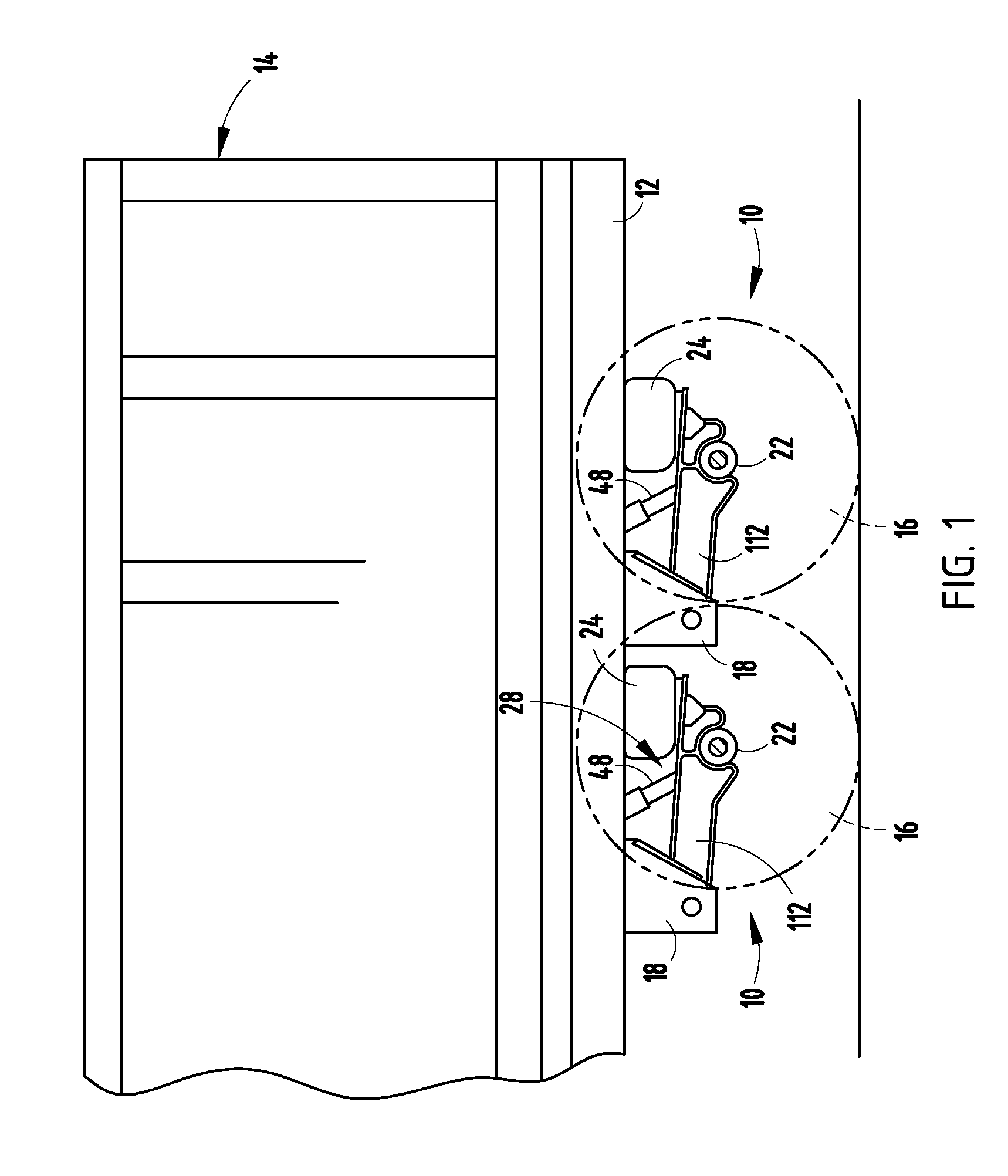
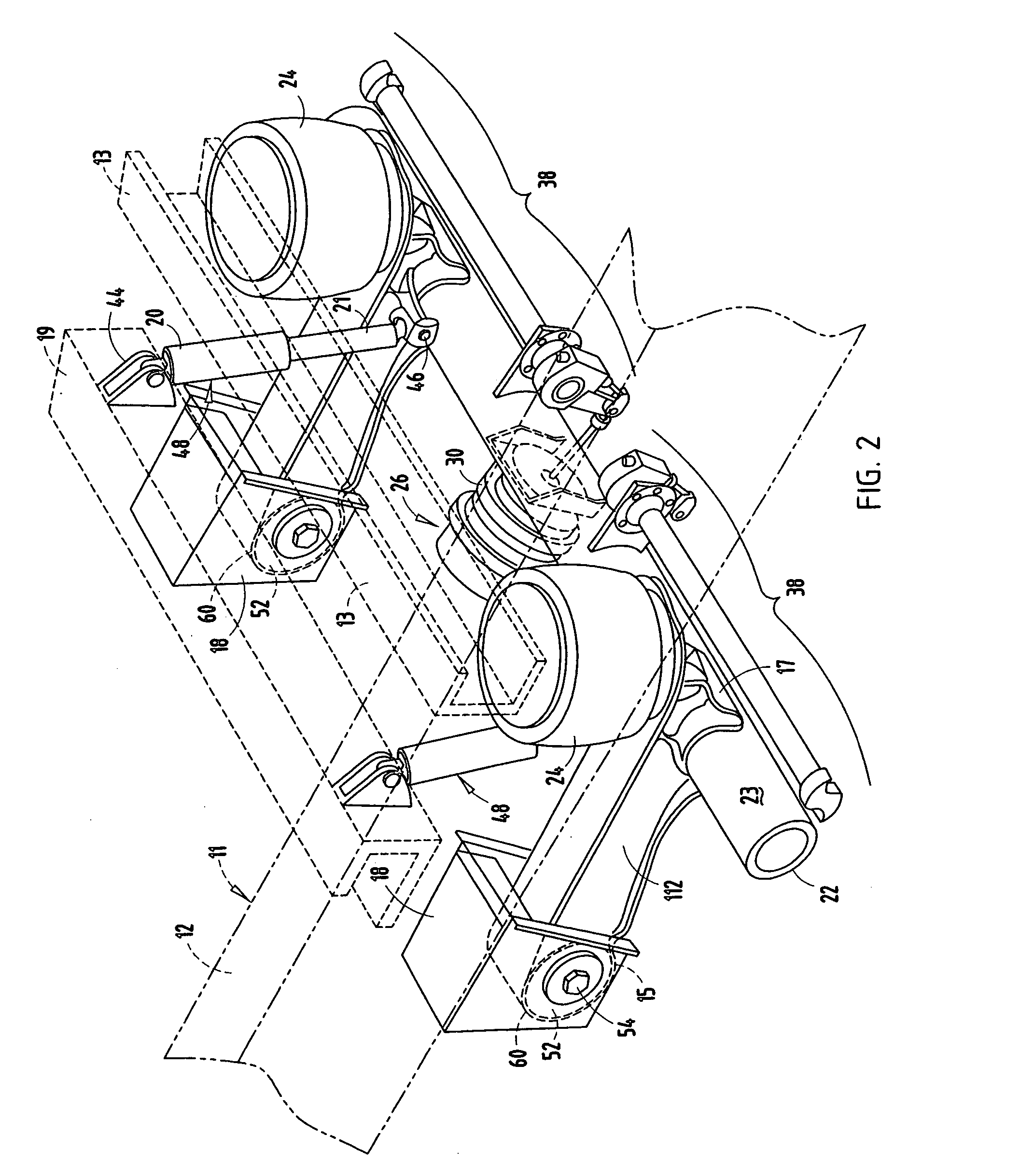
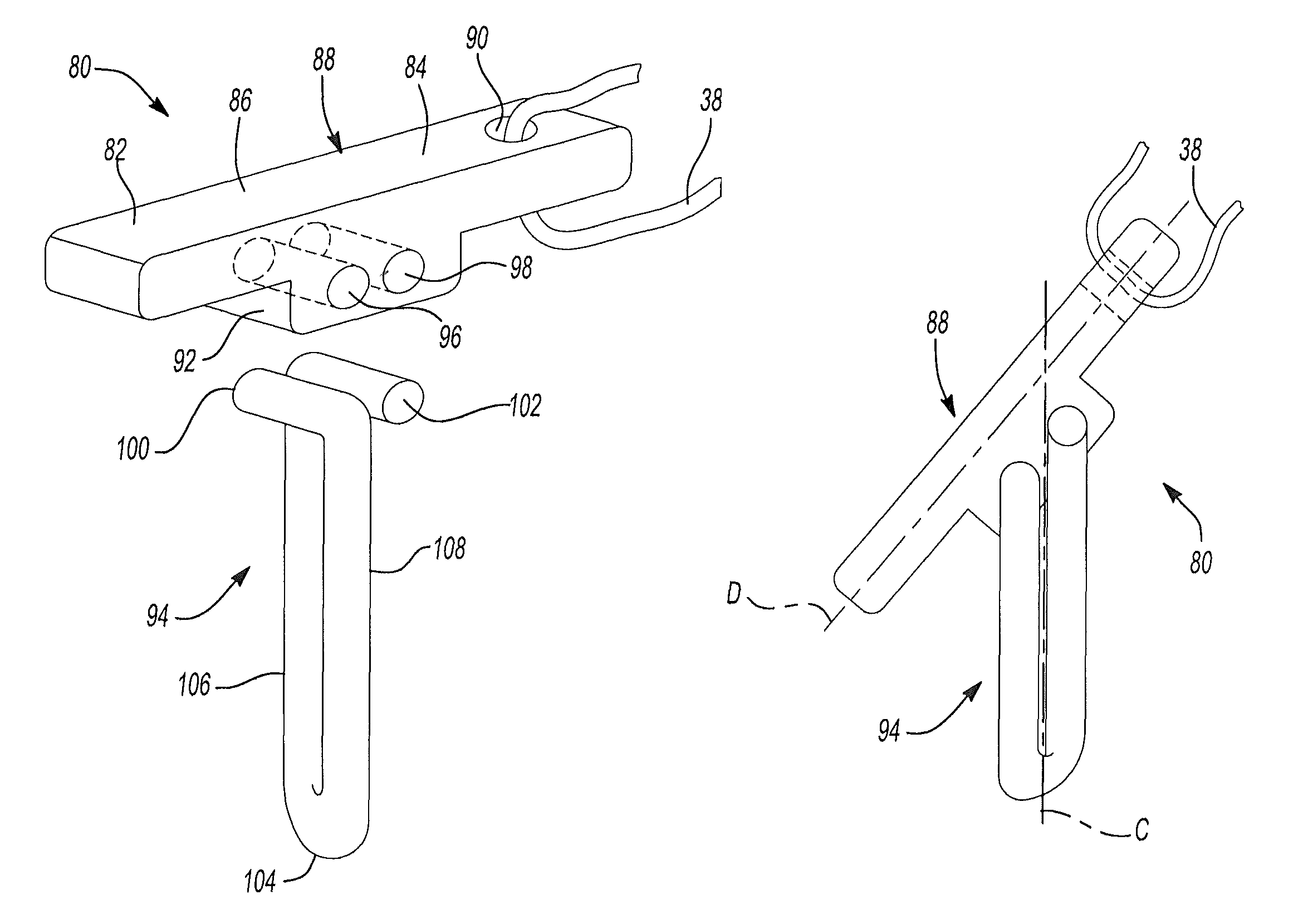
Retention apparatus for an external portion of a semi-implantable hearing aid
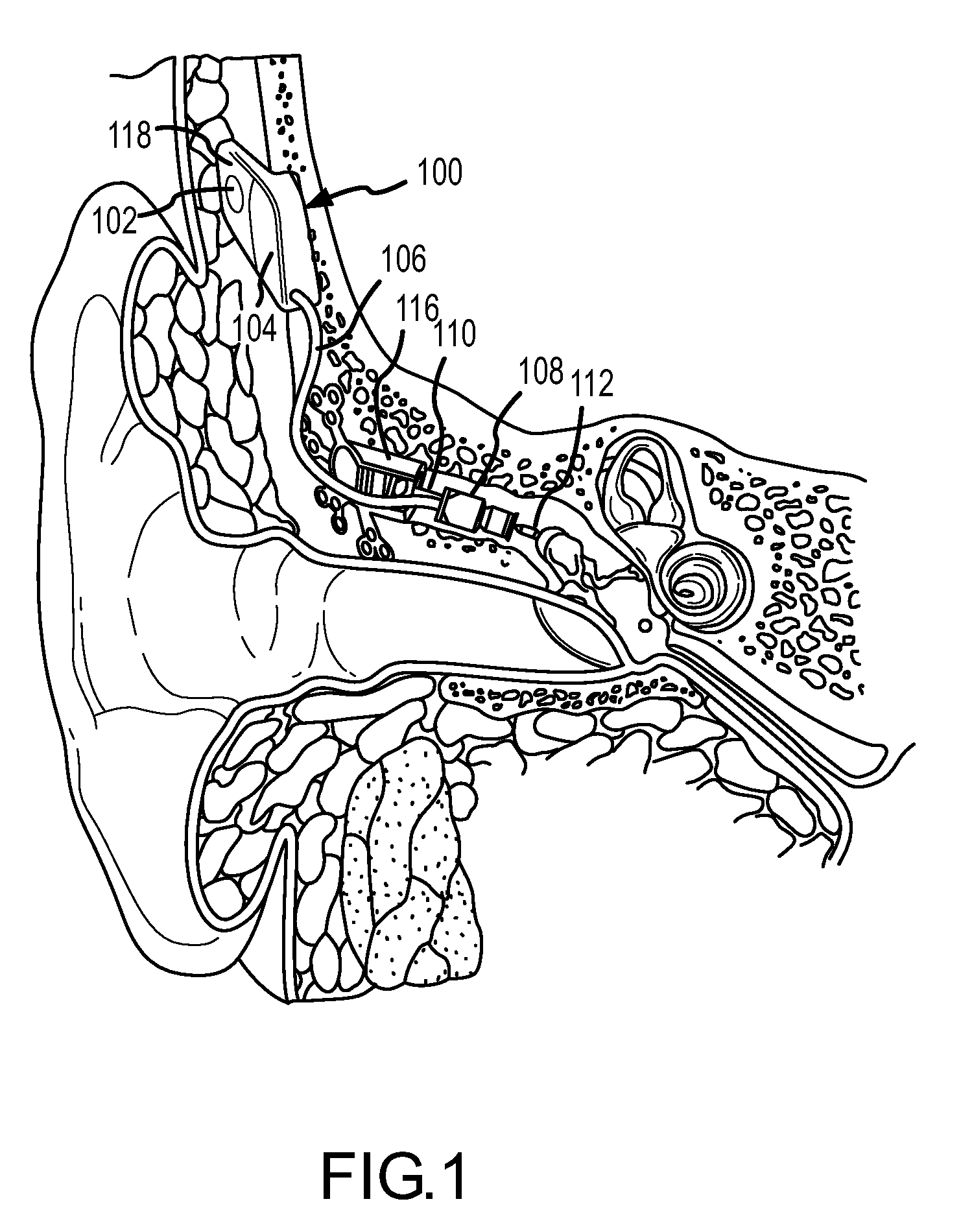
ActiveUS7386143B2Easy to installIncrease magnetic attractionElectrotherapyDeaf-aid setsHearing aidBiomedical engineering
A retention apparatus for a semi-implantable hearing aid. In one embodiment, the retention apparatus includes a first surface and a second surface. One of the first and second surfaces includes a first portion and a second portion having a rounded transition therebetween for interfacing with a patient's skin. The rounded transition of the interfacing surface of the retention apparatus functions to distribute pressure resulting from the mutual magnetic attraction between an externally located magnet and an implanted magnet to permit increased magnetic forces therebetween and maintenance of a desired separation between an external coil and implanted coil.
Owner:COCHLEAR LIMITED
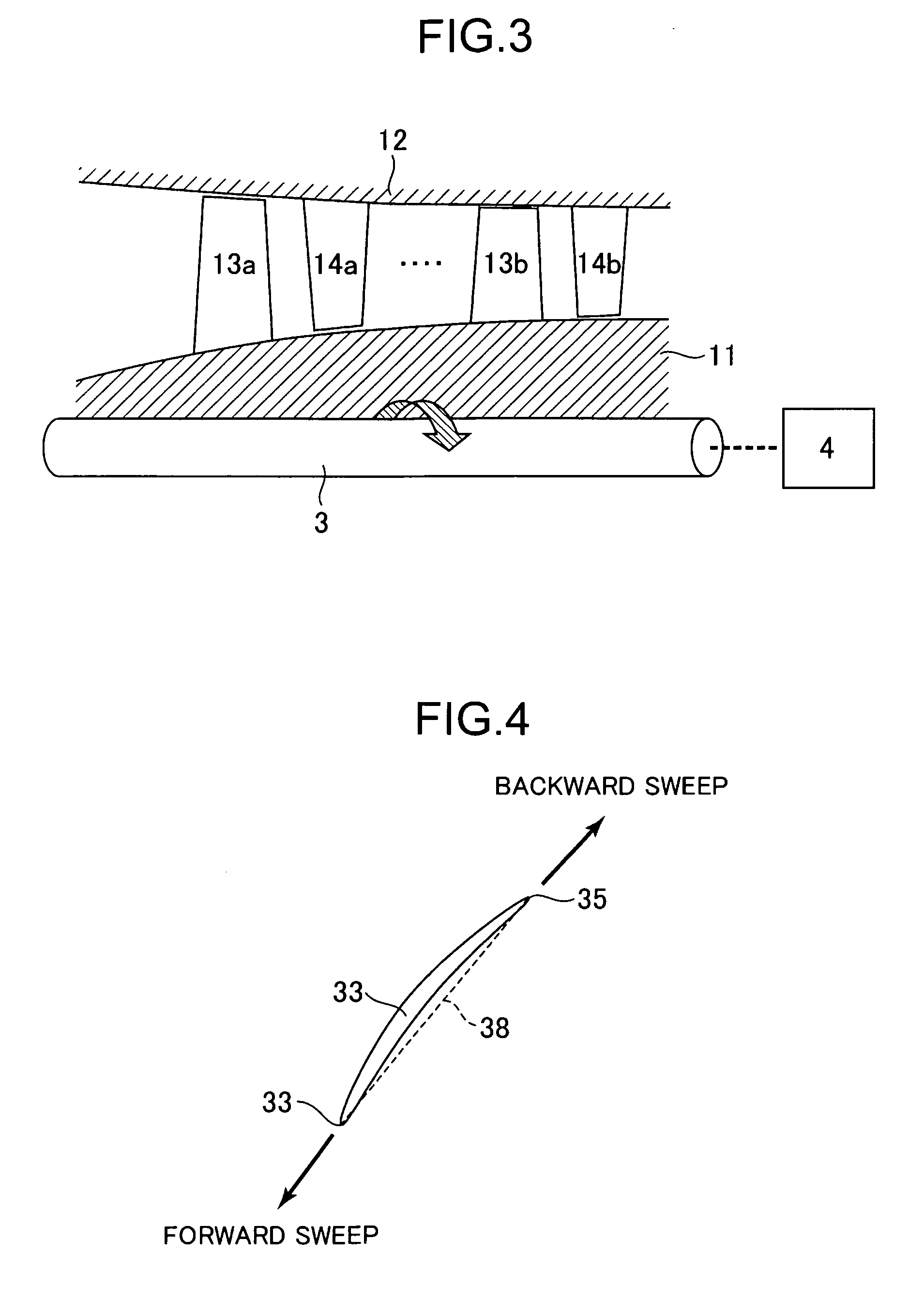
Transonic blade
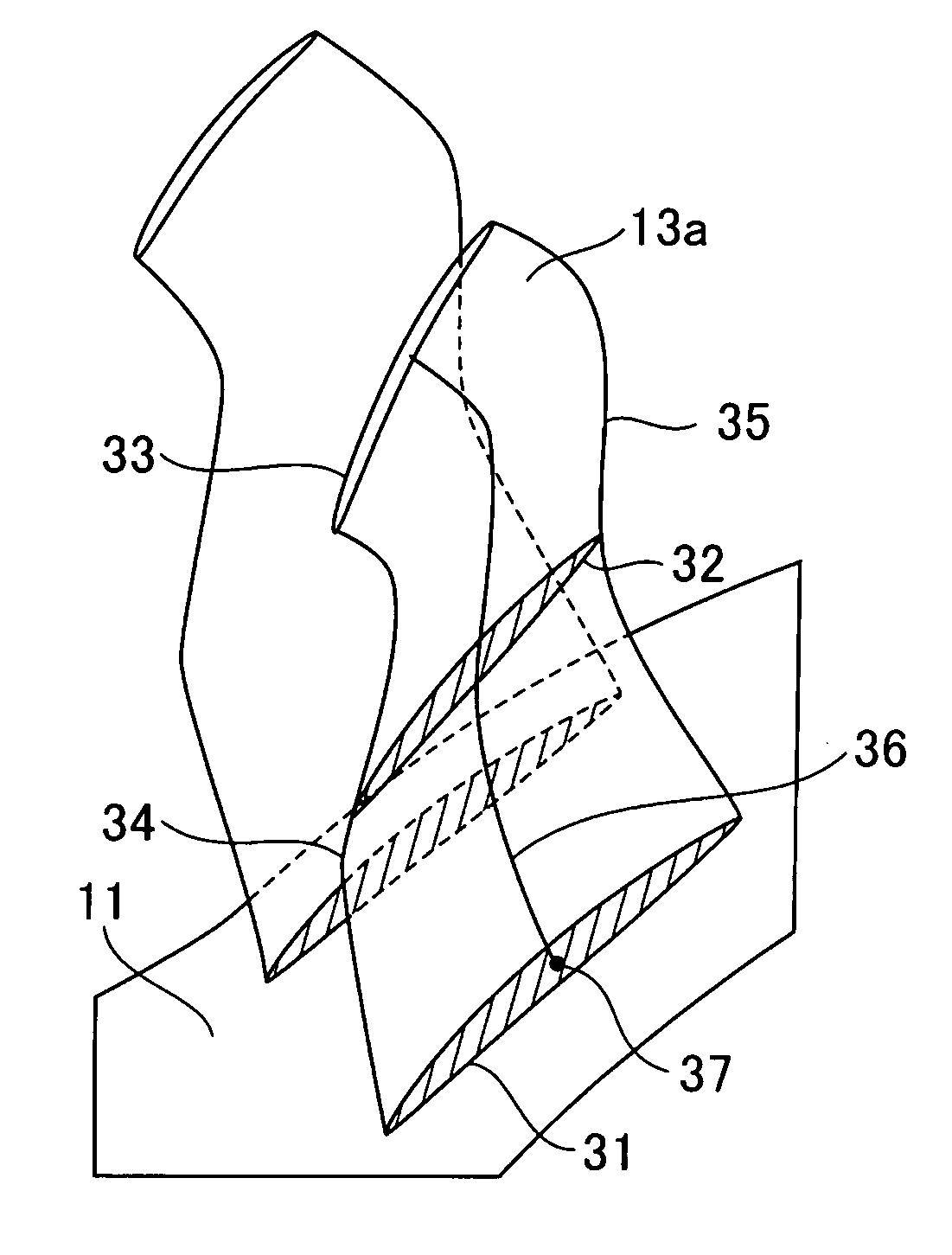
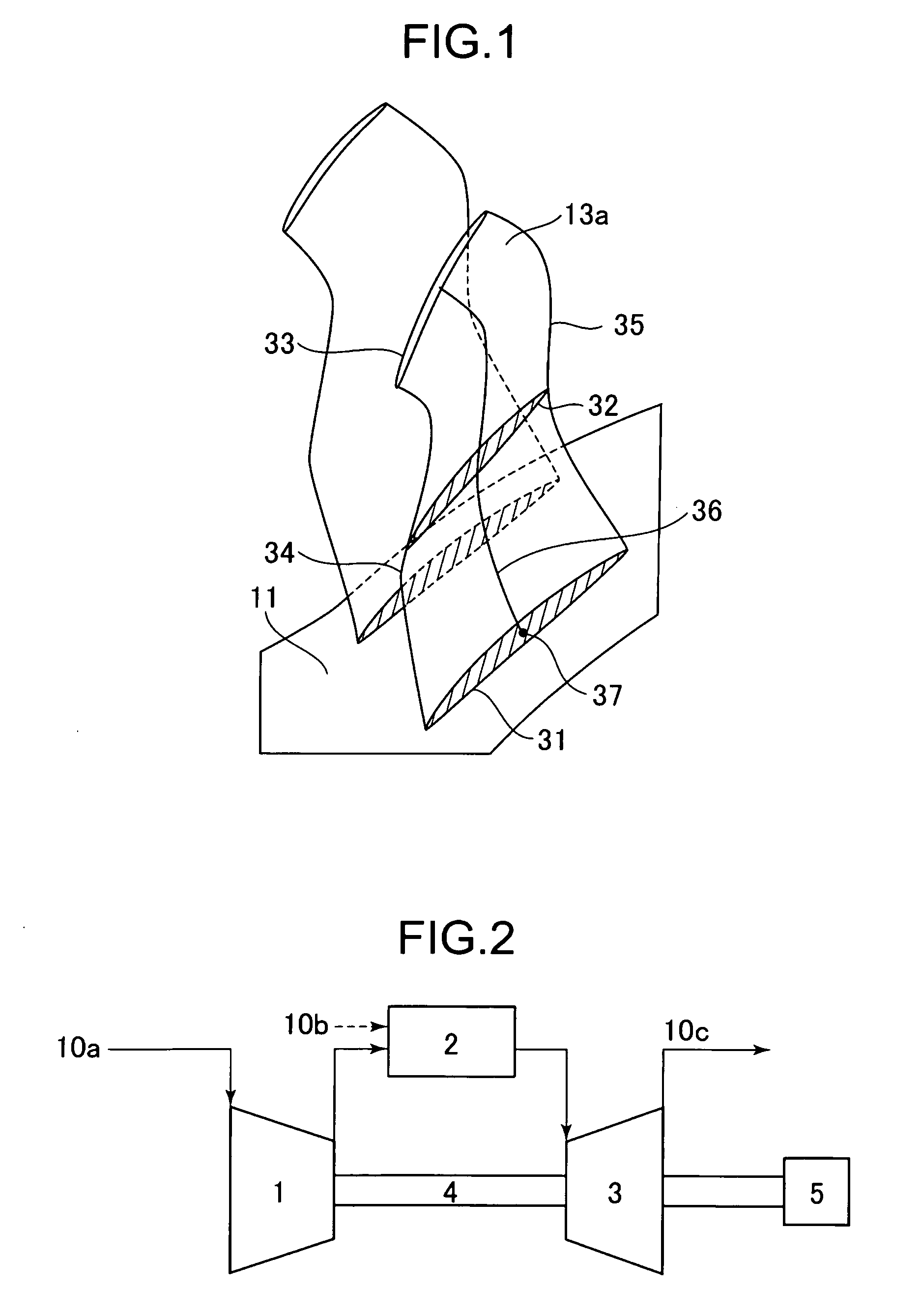
ActiveUS20100215503A1Reduce various lossesImprove local stressPropellersReaction enginesLeading edgeWorking fluid
A transonic blade is provided that operates in a flow field where flow has a transonic speed or higher in an axial-flow rotating machine and that concurrently achieves a reduction in shock loss and in the local stress of the blade.The transonic blade includes a hub cross-sectional surface joined to a rotating shaft or an outer circumferential side casing of a rotating machine; a tip cross-sectional surface located furthest from the hub cross-sectional surface in a spanwise direction which is a vertical direction of the rotating shaft; a leading edge located on an upstream side; and a trailing edge located on a downstream side. At least a part of a passing working fluid flow has a transonic speed or higher. A portion of a stacking line which is a line connecting together respective gravity centers of cross-sectional surfaces located from the hub cross-sectional surface to the tip cross-sectional surface is located on a downstream side of a stacking center in a flow direction of a working fluid main flow.
Owner:MITSUBISHI POWER LTD
Trailing arm suspension with optimized I-beam
ActiveUS7484744B2Reduces amount of contactReduce amount of flexureBearing componentsInterconnection systemsVehicle frameTrailing arm
A suspension system for suspending a vehicle frame above a plurality of ground-engaging wheels includes a wheel-carrying axle comprising a first end a second end, and a pair of frame bracket assemblies each comprising a resiliently-bushed pivotable connection defining a pivot axis, wherein the frame bracket assemblies are operably coupled to opposite sides of the frame bracket, and wherein the resiliently-bushed pivotable connection comprises a substantially cylindrically-shaped bushing. The suspension system also includes a pair of trailing arms each comprising a first end operably coupled to the first end and the second end of the axle, respectively, and a second end comprising an aperture that receives the bushing of one of the frame bracket assemblies therein, wherein the aperture of the second end of each trailing arm is nonsymmetrical, thereby causing a nonsymmetrical compression of the bushing about the pivot axis. The suspension system also includes embodiments wherein the aperture of the second end of each trailing arm is nonuniform, thereby reducing rotation of the bushing with respect to the trailing arm, wherein a mating surface of each trailing arm operably coupled to the first and second end of the axle comprise a cavity, thereby reducing a localized stress transferred from the trailing arm to the axle, wherein the second end of each trailing arm further comprises a lip extending radially outward from the aperture and at least one engagement surface extending radially outward from the lip and adapted to abut a bushing-removal tool, as well as other improvements.
Owner:THE HOLLAND GROUP
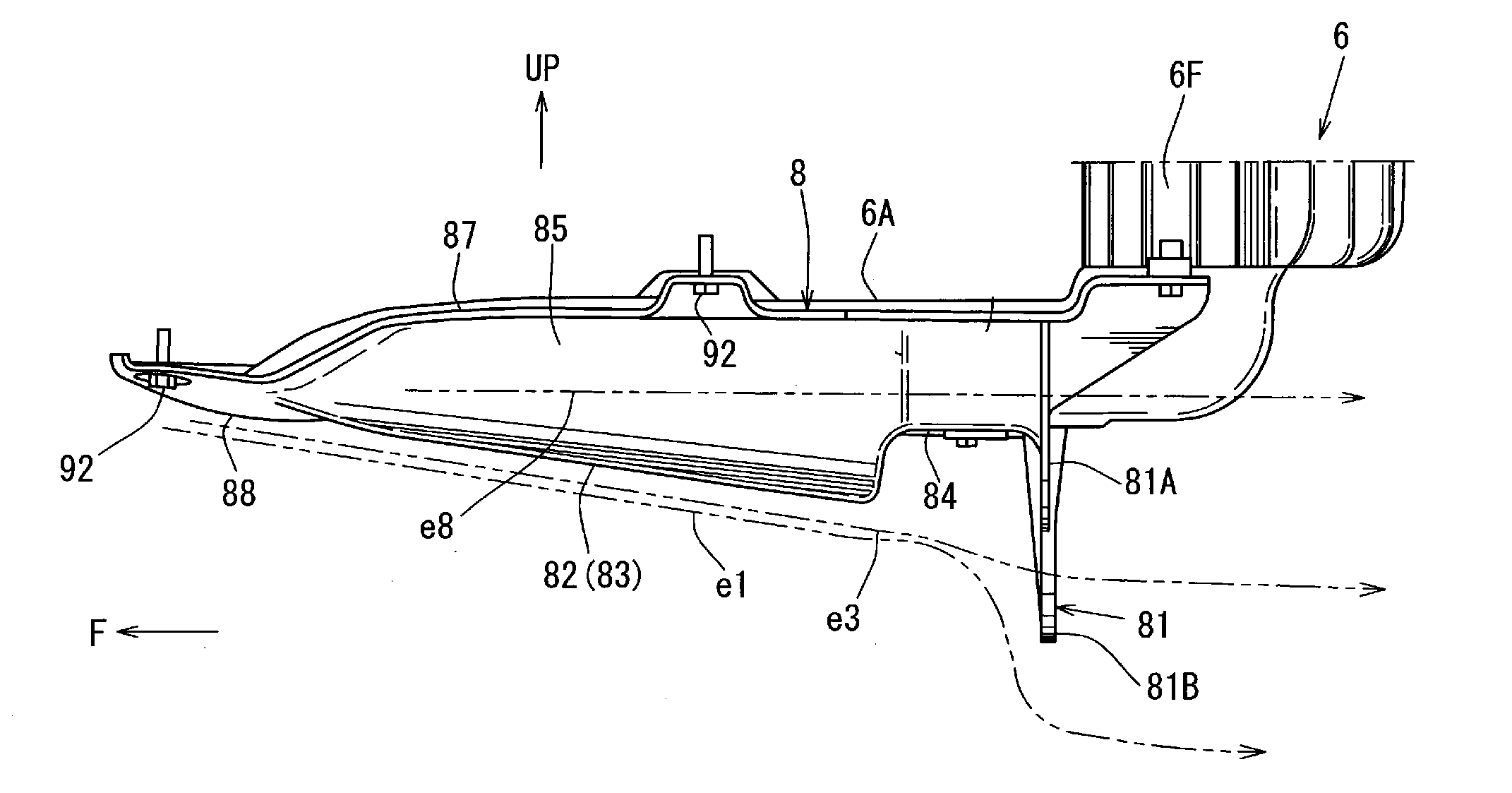
Underfloor structure of automotive vehicle
ActiveUS20160339970A1Reduce air (aerodynamic) resistanceReduce air resistanceVehicle body stabilisationSuperstructure subunitsEngineeringMechanical engineering
Owner:MAZDA MOTOR CORP
Blast effect mitigating assemble using aerogels
ActiveUS20100307327A1Reducing blast wave pressureReduce the average velocityArmoured vehiclesAmmunitionGratingBlast effects
An assembly for protecting against explosions and explosive devices is formed with aerogels and frangible components. The basic configuration forms a space between an object to be protected by an aerogel having a frangible backing layer. Such assemblies may be mounted on vehicles and structures, and alternatively used as barriers without attachment to other objects. Different geometries for the rear surface of the assemblies enhance the ability of deflecting gas produced by explosions away from objects to be protected. Flowable attenuating media may be introduced into the space behind the aerogel and in gratings placed in the front of assemblies in order to increase blast energy dissipation in intense blast conditions. Armor components may be added to the rear surface to protect against fragments and projectiles. Aerogels, metal foams, and dense ceramic beads may be incorporated to enhance protection against explosively-formed penetrators and other projectiles.
Owner:HYBRID COMPONENTS & COATINGS
Semiconductor device and manufacturing method thereof
InactiveUS7808098B2Improve reliabilityNot easy to damageSemiconductor/solid-state device detailsSolid-state devicesFiberInorganic compound
The present invention provides a semiconductor device which is not easily damaged by external local pressure. The present invention further provides a manufacturing method of a highly-reliable semiconductor device, which is not destroyed by external local pressure, with a high yield. A structure body, in which high-strength fiber of an organic compound or an inorganic compound is impregnated with an organic resin, is provided over an element substrate having a semiconductor element formed using a single crystal semiconductor region, and heating and pressure bonding are performed, whereby a semiconductor device is manufactured, to which the element substrate and the structure body in which the high-strength fiber of an organic compound or an inorganic compound is impregnated with the organic resin are fixed together.
Owner:SEMICON ENERGY LAB CO LTD
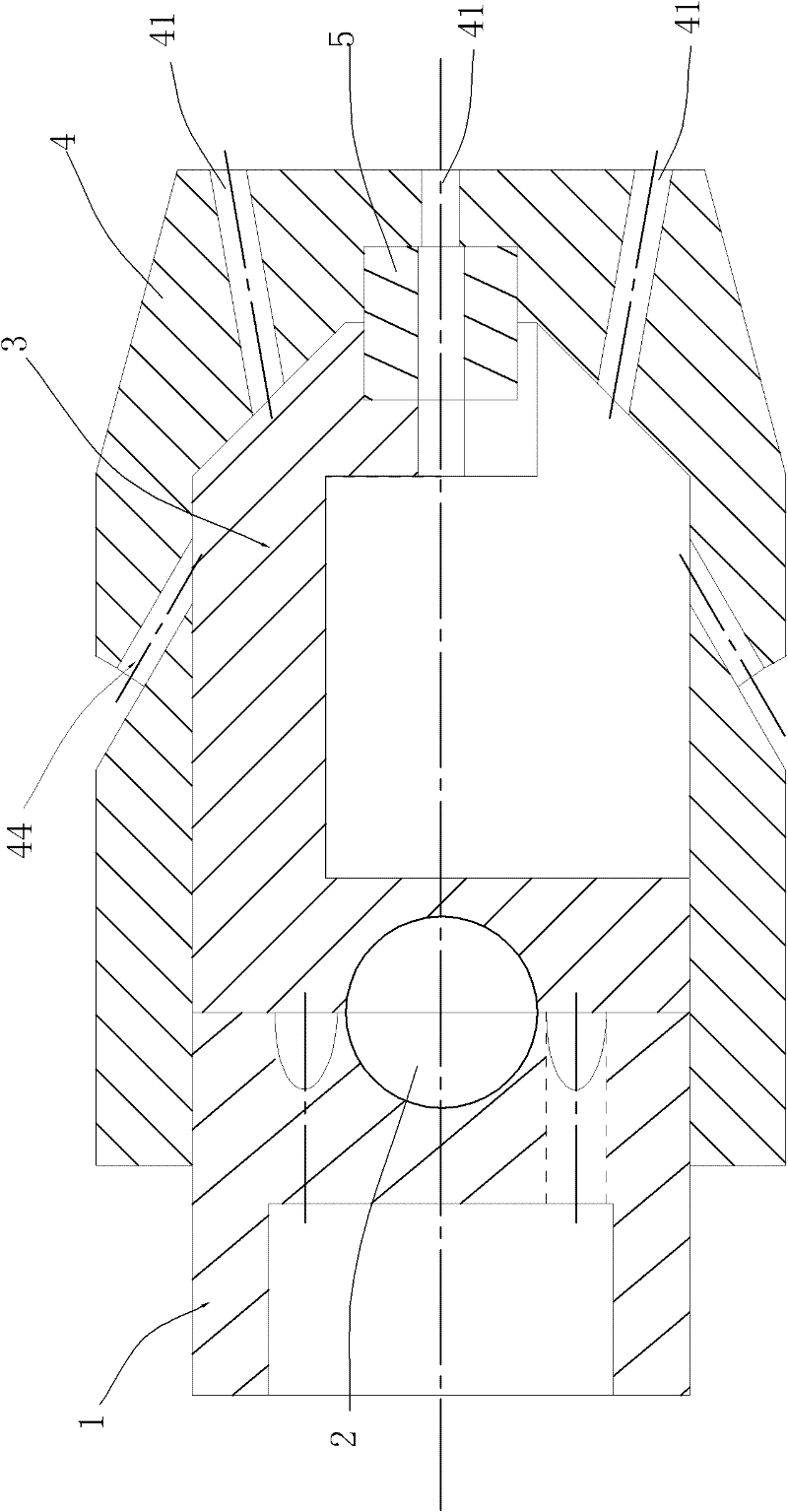
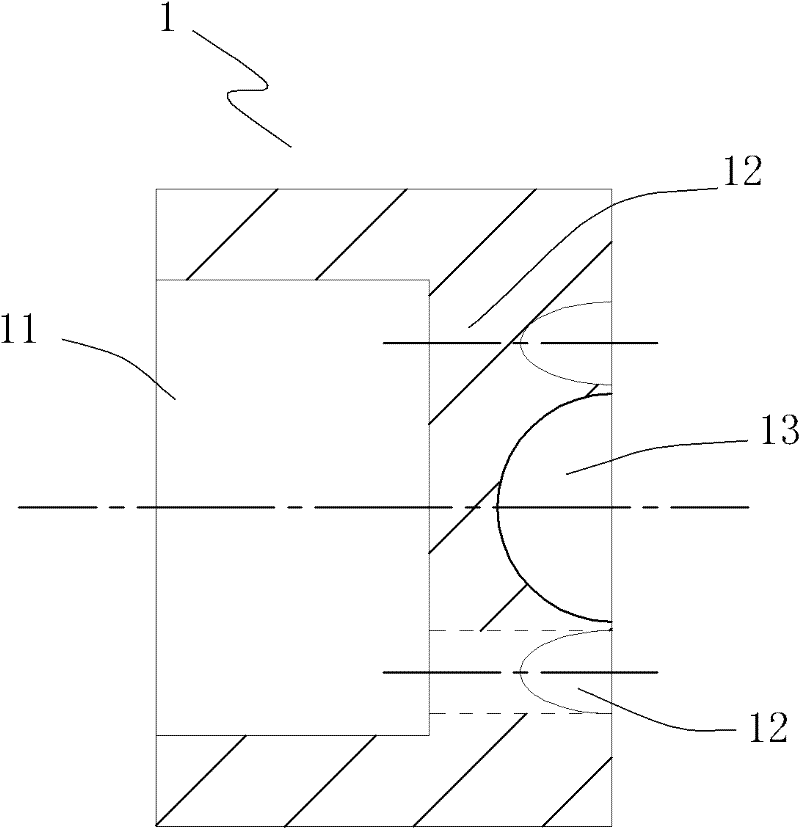
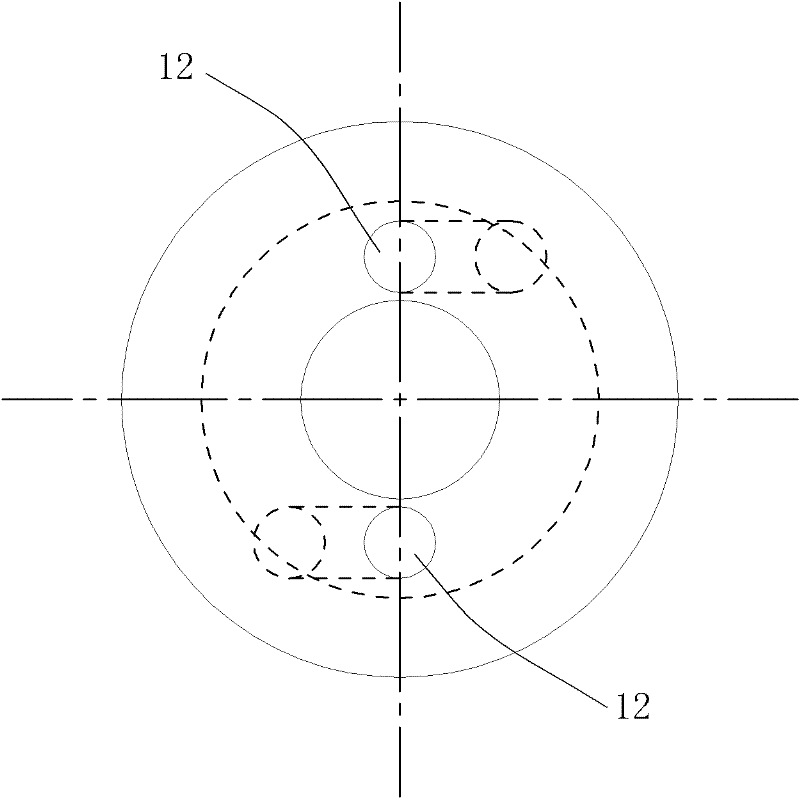
Self-propelled drilling method and pulsed cavitation swirling jet nozzle
The invention relates to a self-propelled drilling method and a nozzle. The self-propelled drilling method is formed by coupling a pulsed jet method, a cavitation jet method and a swirling jet method, wherein, an impeller body arranged in the nozzle can rotate under the impact action of a fluid, a part of forward jet orifices arranged at the head of the nozzle as well as a part of backward jet orifices which are arranged on the nozzle and are opposite to the forward jet orifices can be closed during the rotation process of the impeller body, the fluid is ejected from the other part of the forward jet orifices and the backward jet orifices so as to form jet flow, and the forward jet orifices are alternately closed so as to generate pulsed jet during the rotation process of the impeller body; the fluid entering the nozzle is driven to rotate by the rotating impeller body, and then the rotating fluid is ejected from the jet orifices so as to generate swirling jet; and the central pressure in the nozzle can be reduced by means of rotation of the impeller body so as to form cavitation jet. In the self-propelled drilling method, advantages of the three types of jet flow are integrated so as to greatly improve the drilling efficiency; and the self-propelled drilling method and the nozzle are applicable to drilling holes in a ground layer.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
Bonnet with spandex elastic strip
A head covering bonnet with an opening for the head and an elastic spandex band below the opening for holding the bonnet to the head. The spandex band is of a width so as not to apply high localized pressure on the hair, and spandex is not rough or abrasive when stretched, unlike typical elastic material which becomes rough and possibly abrasive to hair when the elastic is stretched.
Owner:SPARTAN BRANDS
Pulse cavitation multiple jet nozzle
ActiveCN102434102ASimple structureEasy to manufactureSpray nozzlesLiquid/gas jet drillingImpellerCavitation
The invention relates to a pulse cavitation multiple jet nozzle, which comprises a nozzle body which is formed into an open end on one end and a jet end with a hollow cylinder on the other end, the open end is used for connecting with a fluid pipe, and the jet end is provided with a plurality of jet orifices; the central position of the jet end is provided with a central jet orifice, and a plurality of lateral jet orifices are arranged around the central jet orifice; the nozzle body is internally provided with an impeller being capable of rotating, a part of the fluid flowing into the nozzle passes through the center of the impeller and the central jet orifice to form continuous straight jet, the other part of the fluid impacts the blades of the impeller to cause the impeller to rotate so as to make flow field on the inlets of the lateral jet orifices around the central jet orifice generate regular disturbance, thus a pulse jet can be formed, vacuole can be produced, a cavitation jet can be formed, and the cavitation jet and the pulse jet are coupled to form a pulse cavitation jet to be ejected from the lateral jet orifices. The invention is simple in structure, and can greatly improve the efficiency of drilling.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
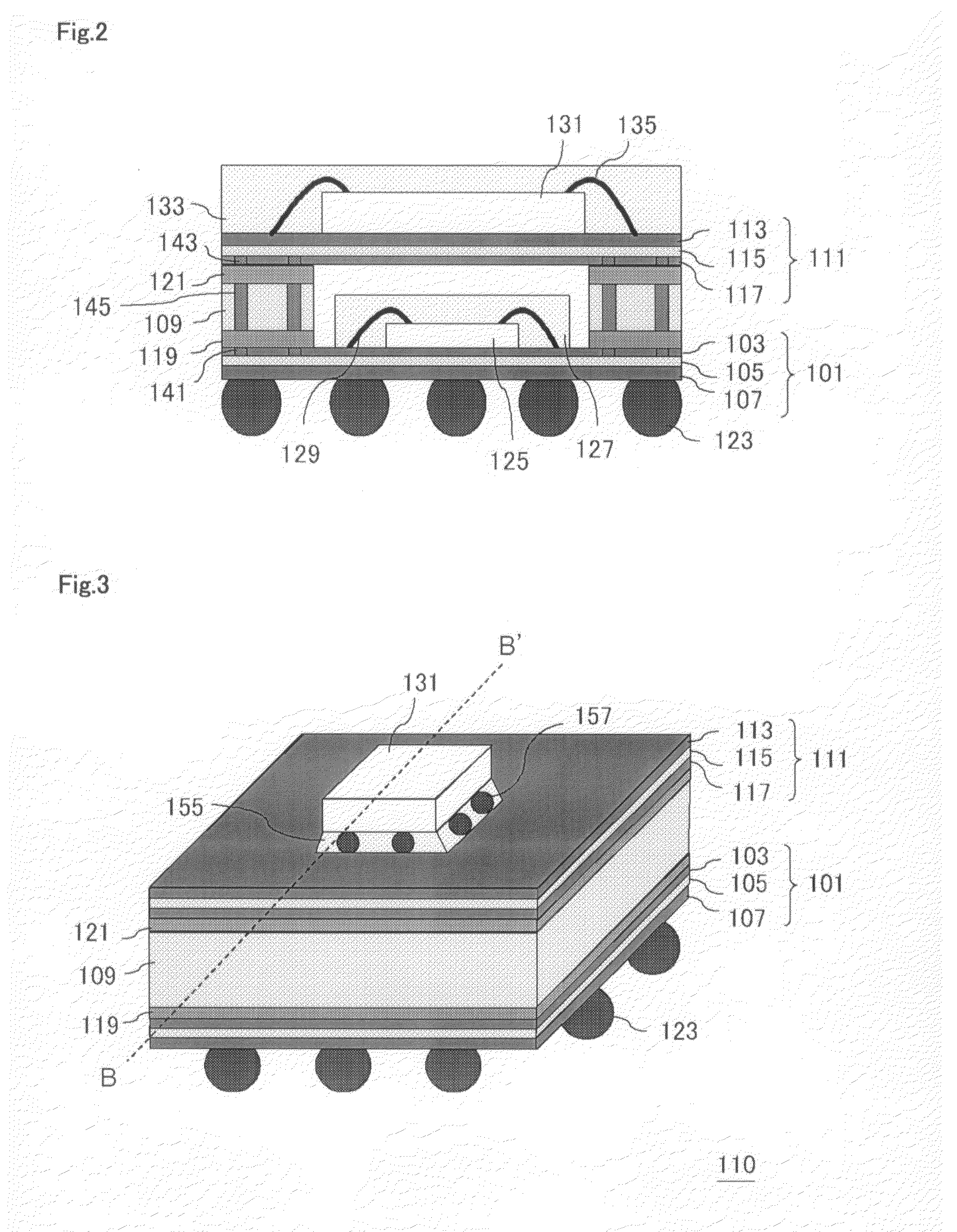
Semiconductor device and method for manufacturing semiconductor device
InactiveUS7829992B2Inhibition is effectiveImprove joint reliabilitySemiconductor/solid-state device detailsSolid-state devicesSurface mountingSemiconductor chip
A semiconductor device (100) comprises a first resin substrate (101) on which a first semiconductor chip (125) is mounted a surface thereof; a second resin substrate (111) on which a second semiconductor chip (131) is mounted on a surface thereof; and a resin base material (109), joined to a front surface of the first resin substrate (101) and to a back surface of the second resin substrate (111), so that these surfaces are electrically connected. The resin base material (109) is disposed in a circumference of the first resin substrate (101) in the surface of the first resin substrate (101). Further, the first semiconductor chip (125) is disposed in a space section provided among the first resin substrate (101), the second resin substrate (111) and the resin base material (109) in the surface of the first resin substrate (101).
Owner:SUMITOMO BAKELITE CO LTD
Package with strap handle
InactiveUS20090277916A1Sufficient widthImprove comfortSealingContainers preventing decayEngineeringMechanical engineering
A package, and method of manufacturing the same, the package having an attached strap handle for ease in carrying and pouring of the contents. In one embodiment, the package includes a pair of panels separated by a gusseted panel portion. Each end of the strap handle is attached to the package through slits that are formed at the confluence of the gusset panel portion and a respective one of the panels. Seams or seals may be formed to effectively seal the slits and may bond the handles to the package. The handle may additionally include first and second strap handles, and a strap or member spanning between the first and second strap handles.
Owner:STEELE MARK
Local administration of a combination of rapamycin and cilostazol for the treatment of vascular disease
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Fluidic drive
InactiveUS20050066810A1Shorten the lengthEasy to controlFlexible wall reciprocating enginesFluid-pressure actuatorsEngineeringRing element
In a fluidic drive for disposition between two components which are movable relative to each other comprising a hollow body with a duct for supplying fluid to, and discharging it from, the hollow body wherein the hollow body consists at least partially of a bellows structure, non-resilient ring elements extend around the hollow body in each of its pleats and a connecting structure extends at least at one side of the hollow body and interconnects the non-resilient ring elements.
Owner:GESELLSCHAFT FUR SCHWERIONENFORSCHUNG GMBH
Manufacturing method of semiconductor device
InactiveUS7968427B2Improve reliabilityNot easy to damageSemiconductor/solid-state device detailsSolid-state devicesFiberInorganic compound
The present invention provides a semiconductor device which is not easily damaged by external local pressure. The present invention further provides a method for manufacturing a highly-reliable semiconductor device, which is not destructed by external local pressure, with a high yield. A structure body, in which high-strength fiber of an organic compound or an inorganic compound is impregnated with an organic resin, is provided over an element layer having a semiconductor element formed using a non-single crystal semiconductor layer, and heating and pressure bonding are performed, whereby a semiconductor device is manufactured, to which the element layer and the structure body in which the high-strength fiber of an organic compound or an inorganic compound is impregnated with the organic resin are firmly fixed together.
Owner:SEMICON ENERGY LAB CO LTD
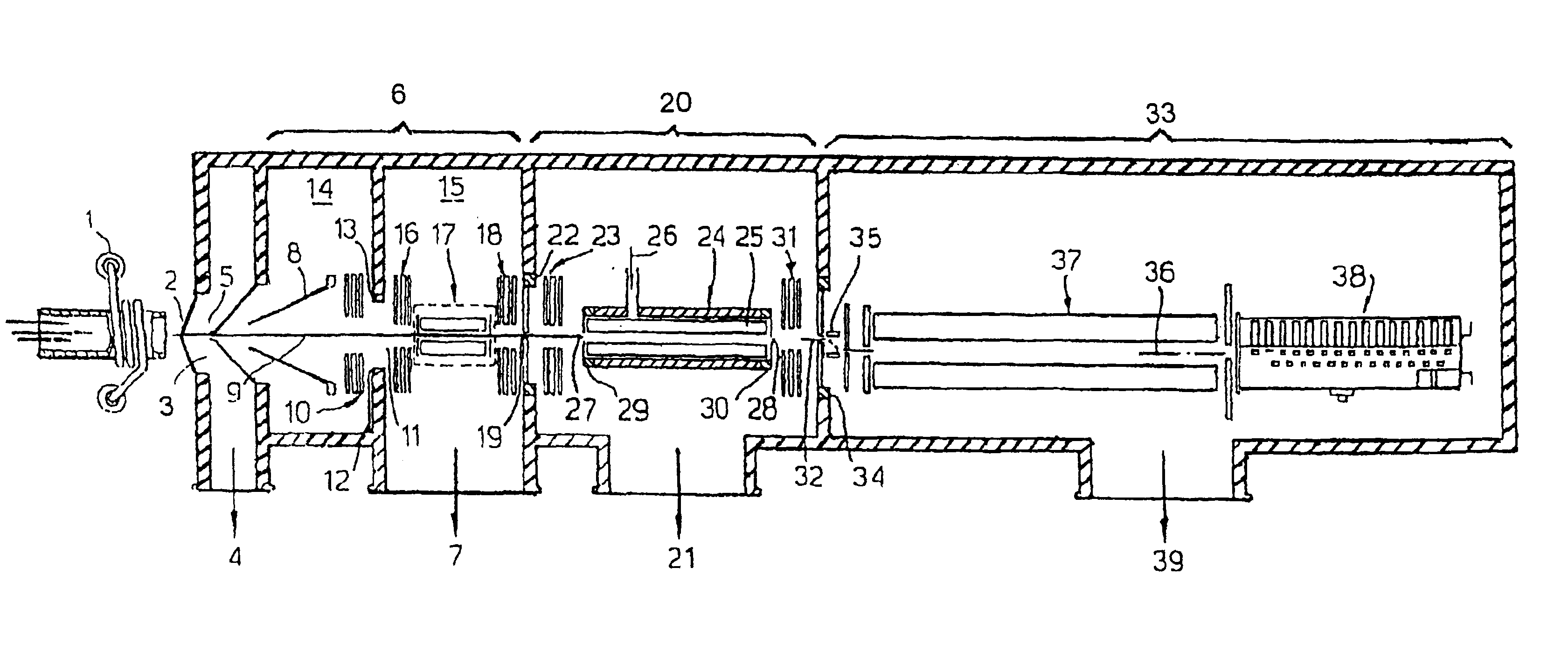
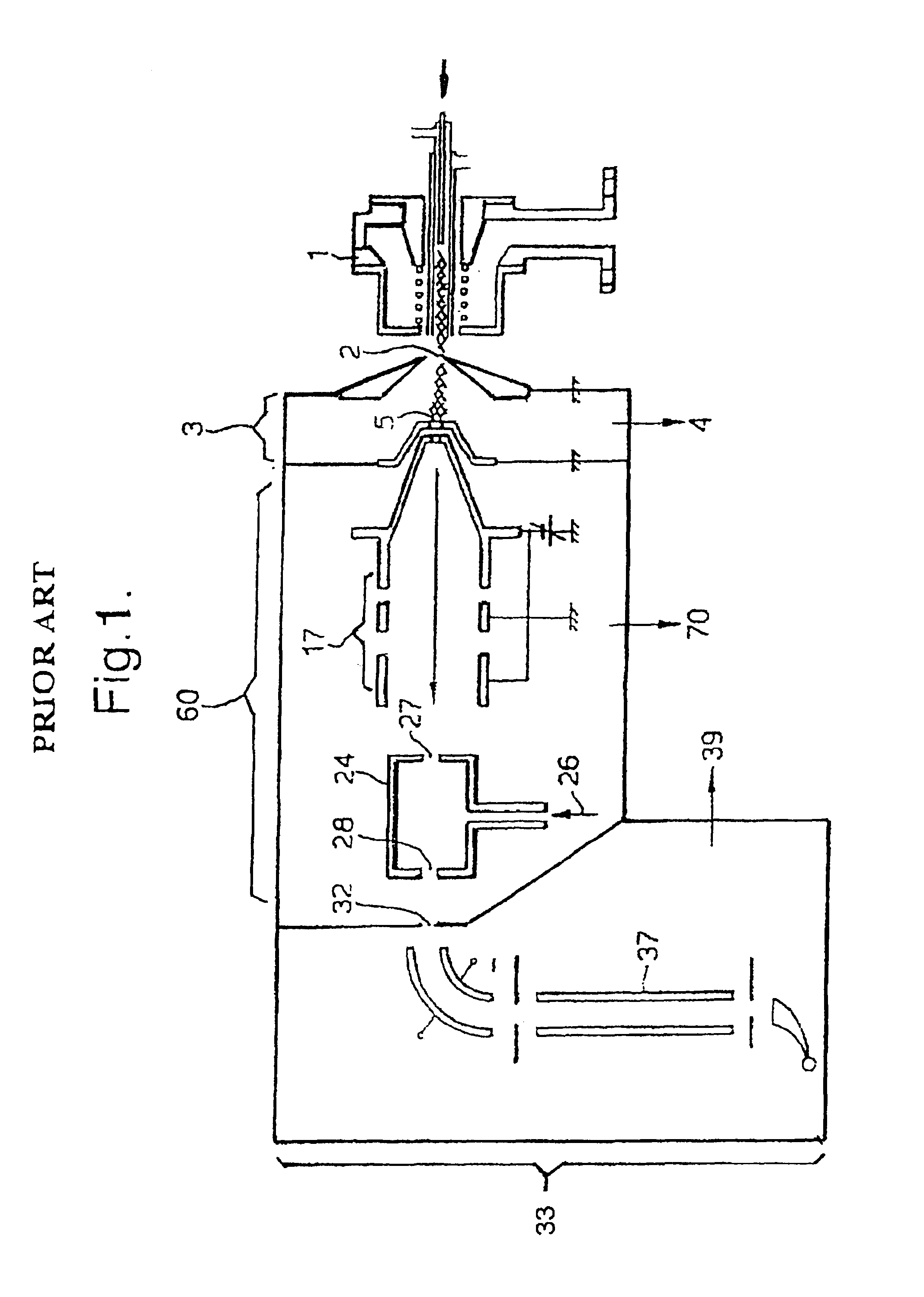
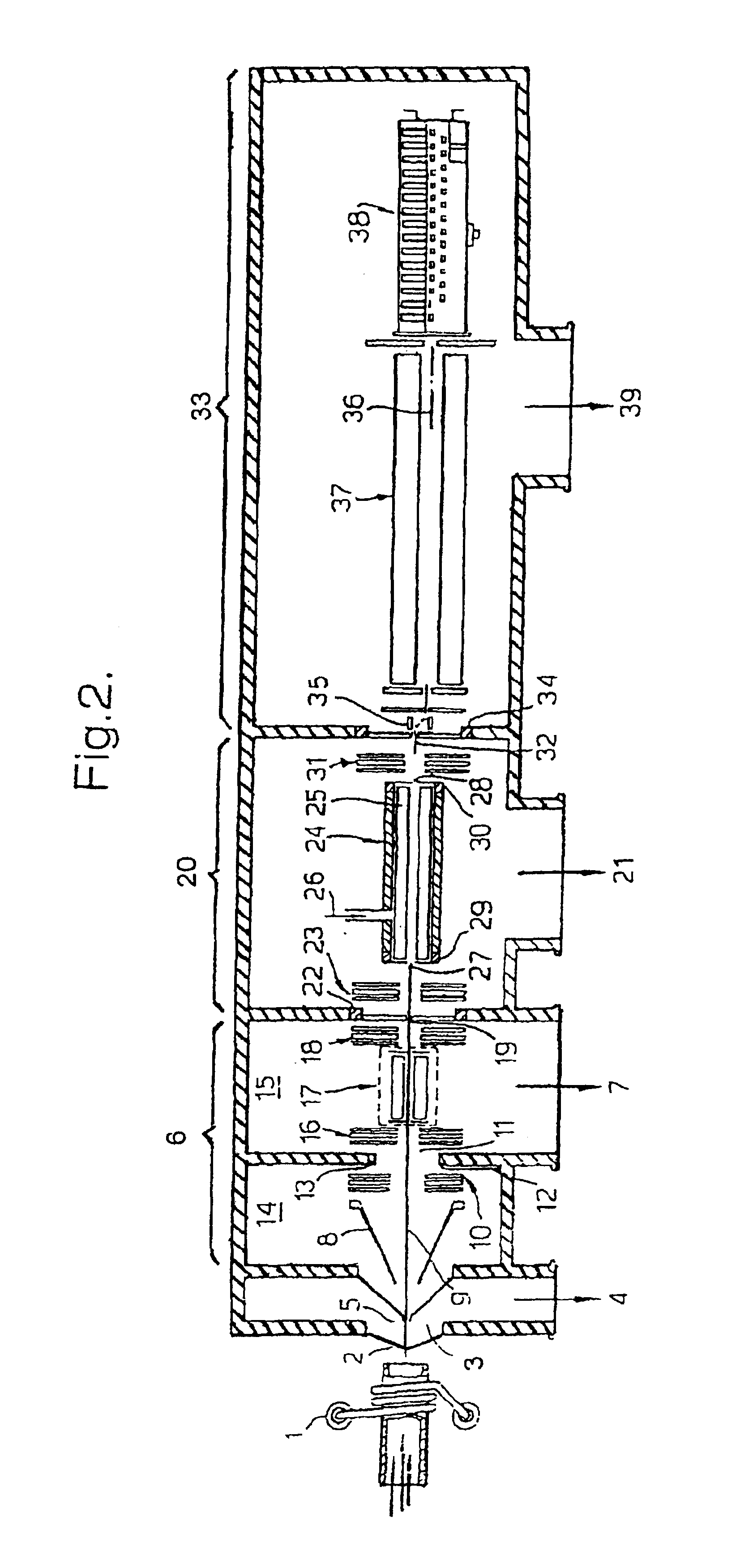
Means for removing unwanted ions from an ion transport system and mass spectrometer
InactiveUS7202470B1Reduce gas loadSufficient transmissionStability-of-path spectrometersSamples introduction/extractionTransport systemIon beam
The present invention relates to inductively coupled plasma mass spectrometry (ICPMS) in which a collision cell is employed to selectively remove unwanted artefact ions from an ion beam by causing them to interact with a reagent gas. The present invention provides a first evacuated chamber (6) at high vacuum located between an expansion chamber (3) and a second evacuated chamber (20) containing the collision cell (24). The first evacuated chamber (6) includes a first ion optical device (17). The collision cell (24) contains a second ion optical device (25). The provision of the first evacuated chamber (5) reduces the gas load on the collision cell (24), by minimising the residual pressure within the collision cell (24) that is attributable to the gas load from the plasma source (1). This serves to minimise the formation, or re-formation, of unwanted artefact ions in the collision cell (24).
Owner:THERMO FISHER SCI BREMEN +1
Method for soft tissue attachment
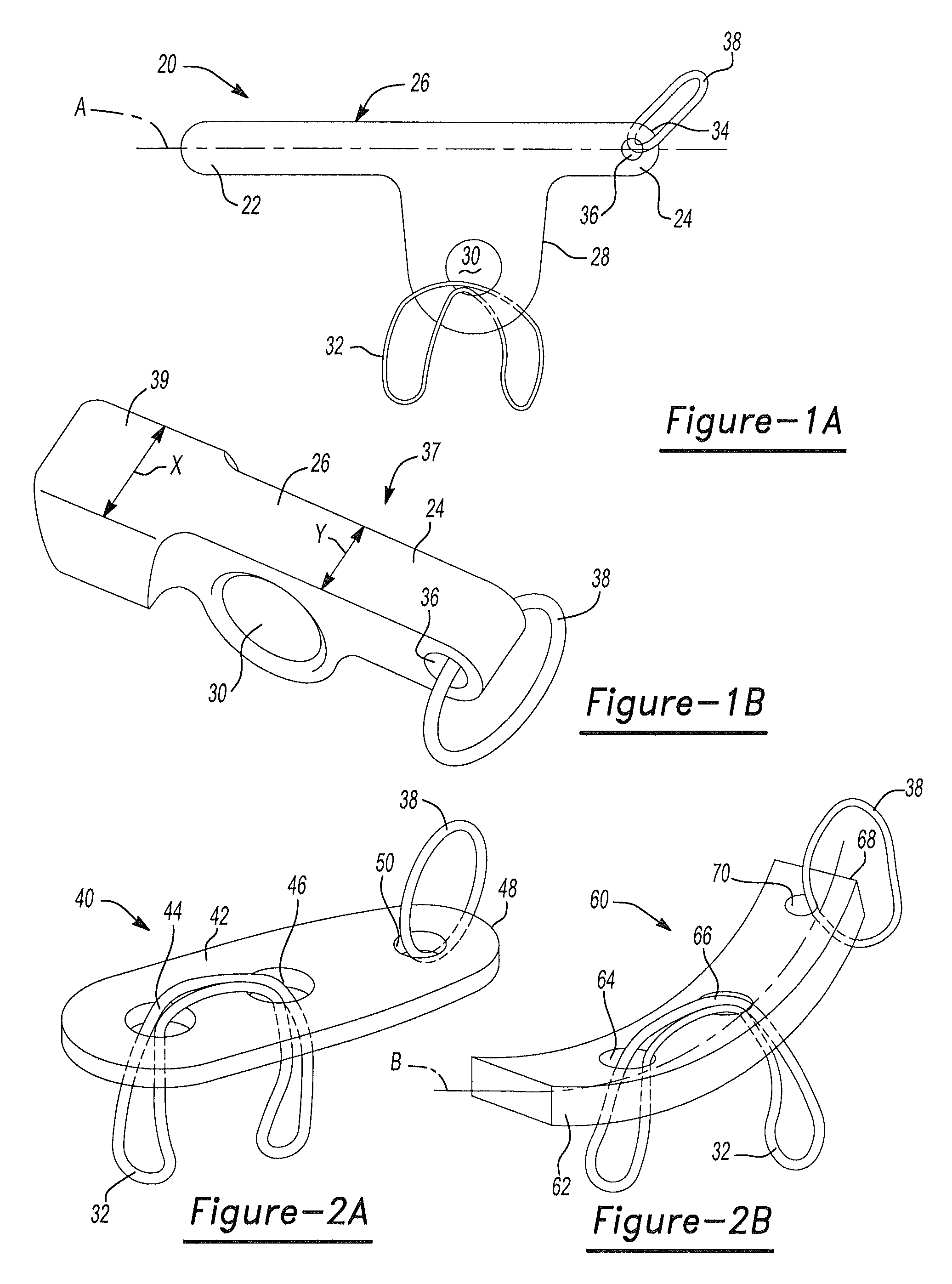
InactiveUS7967843B2Reduce local pressureEasy to implantSuture equipmentsLigamentsGraft portionSoft tissue
An apparatus and method for fixing a selected graft relative to a selected anatomical portion. An anchor may be provided that may be interconnected with a selected graft portion that is operable to pass through a selected bore and then moved into an operable position to engage a selected portion of the bore to substantially eliminate the possibility of the graft moving in an unselected direction through the bore. In addition, a spacer member may be used to expand a selected portion of the graft to reduce localized stress and may increase ingrowth into a selected bony portion.
Owner:BIOMET MFG CORP
Sulfide and oxy-sulfide glass and glass-ceramic films for batteries incorporating metallic anodes
ActiveUS20180294517A1Improving glass formabilityImprove stabilitySolid electrolytesGlass forming apparatusLithium metalElectrical battery
Thin amorphous or partially crystalline lithium-containing and conducting sulfide or oxysulfide glass electrode / separator members are prepared from a layer of molten glass or of glass powder. The resulting glass films are formed to lie face-to face against a lithium metal anode or a sodium metal anode and a cathode and to provide for good transport of lithium ions between the electrodes during repeated cycling of the cell and to prevent shorting of the cell by dendrites growing from the lithium metal or sodium metal anode.
Owner:GM GLOBAL TECH OPERATIONS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com