Patents
Literature
1056results about How to "Reduce compression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
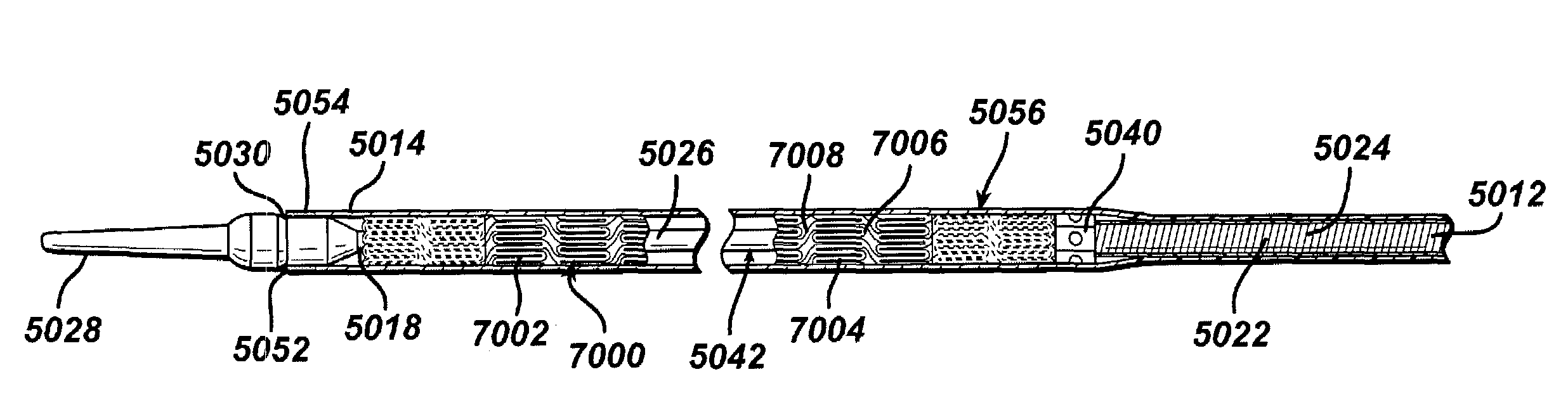
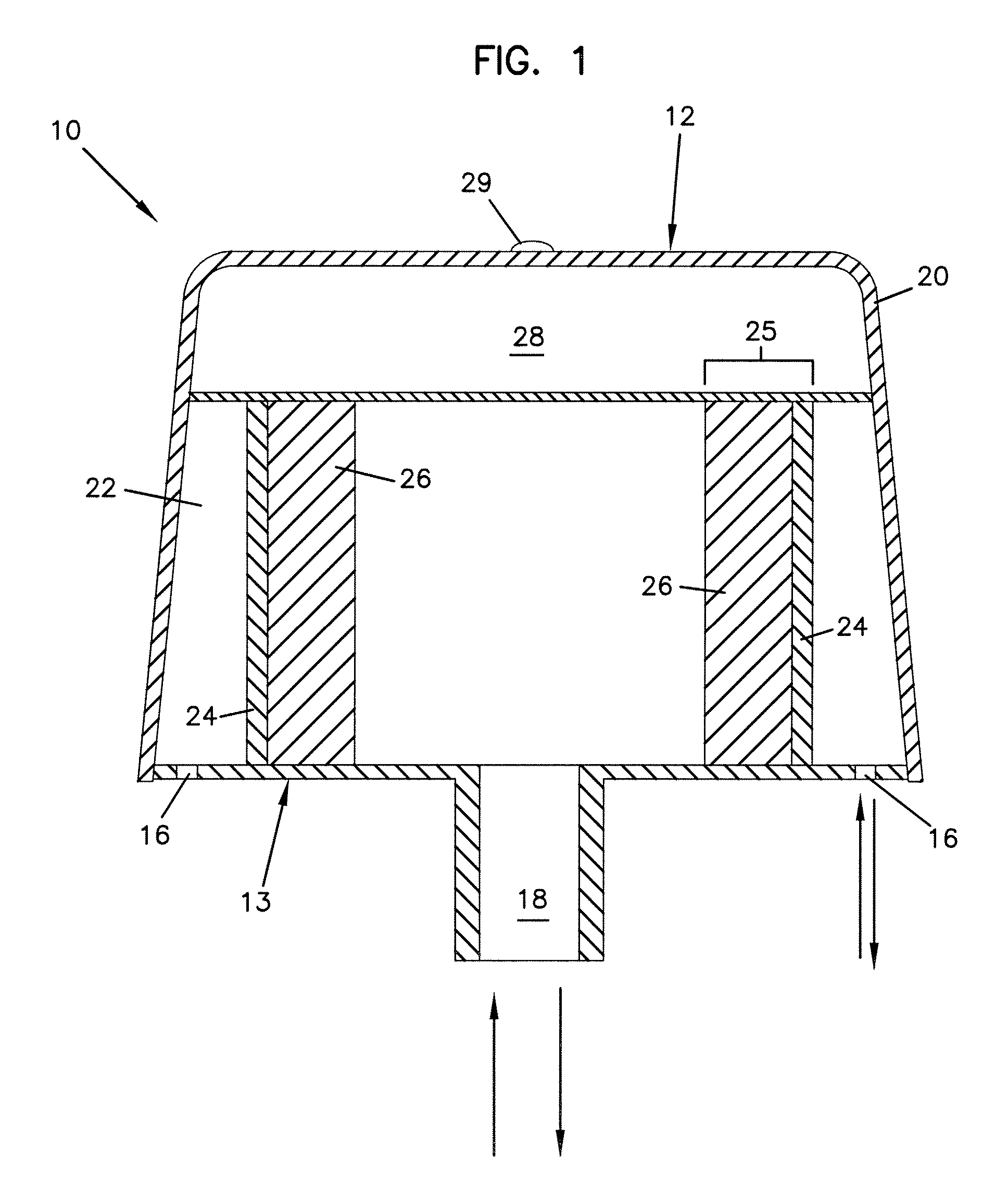
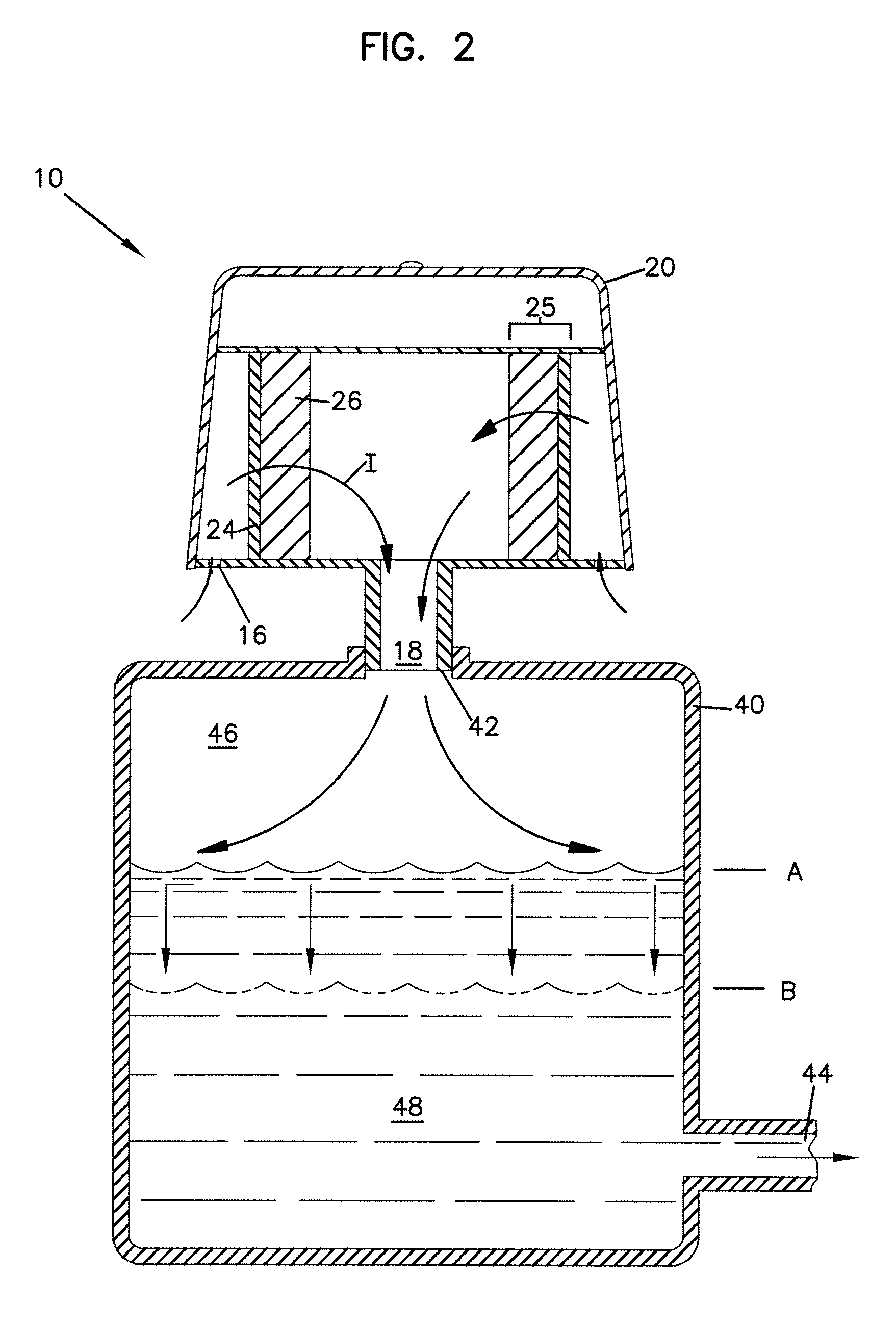
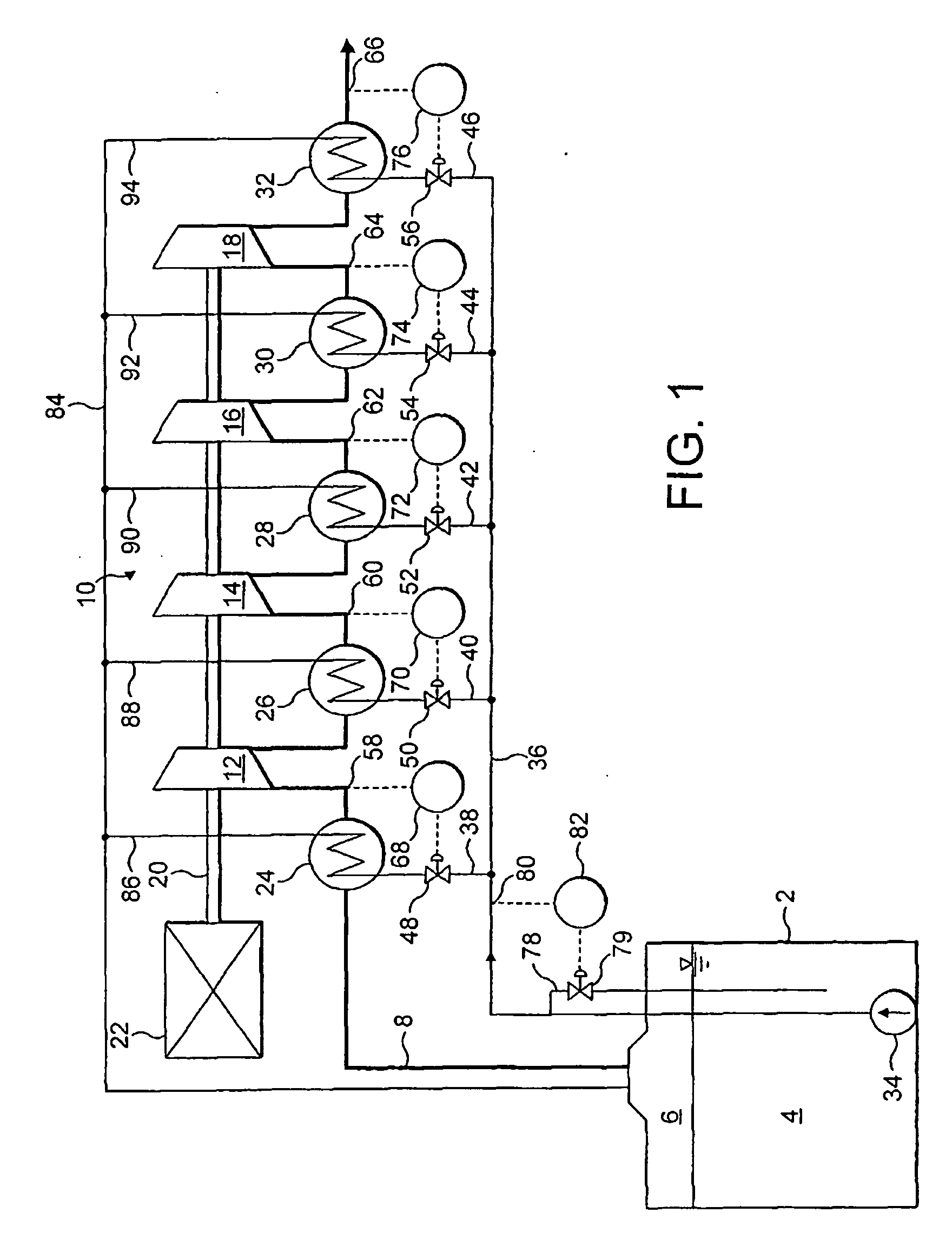
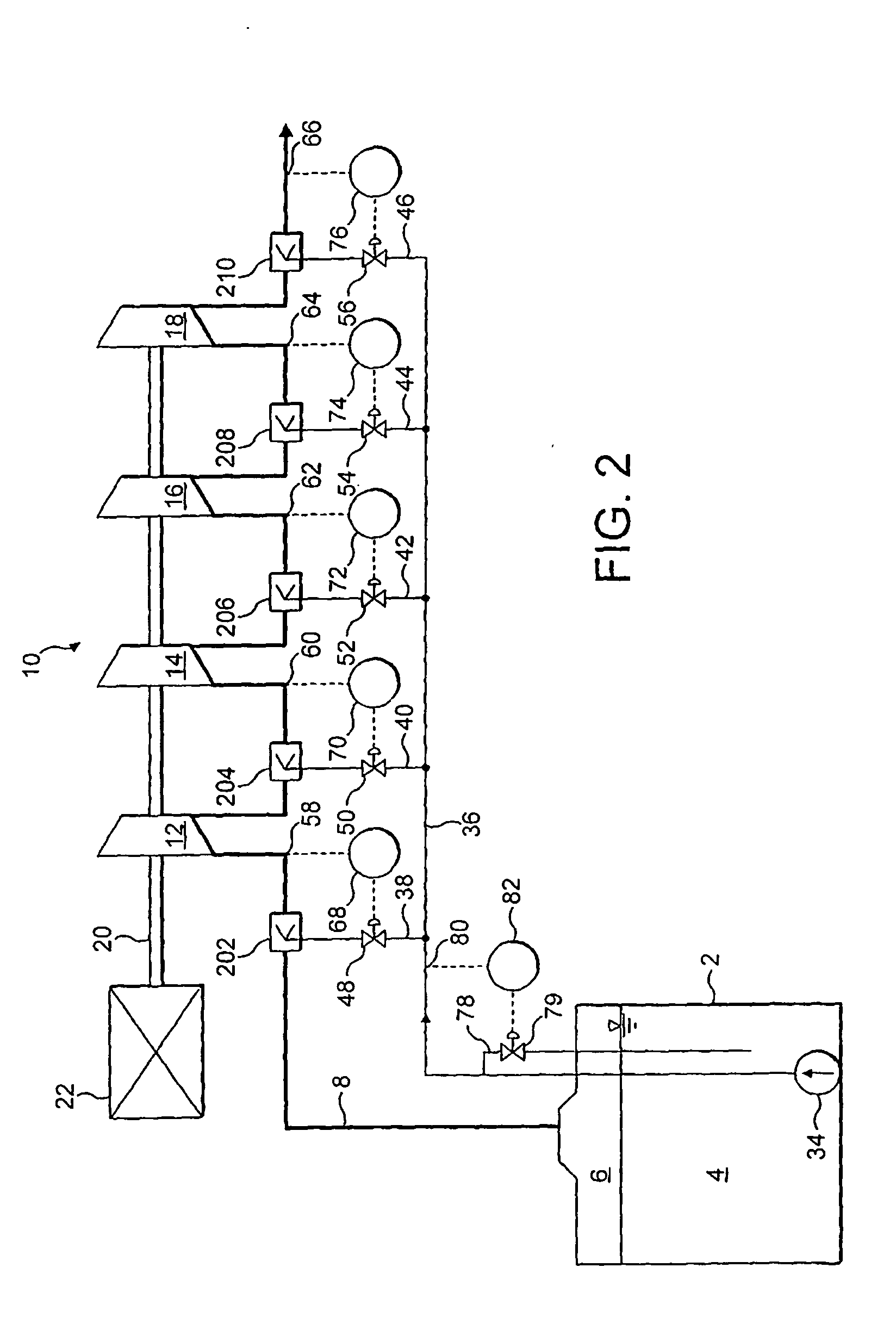
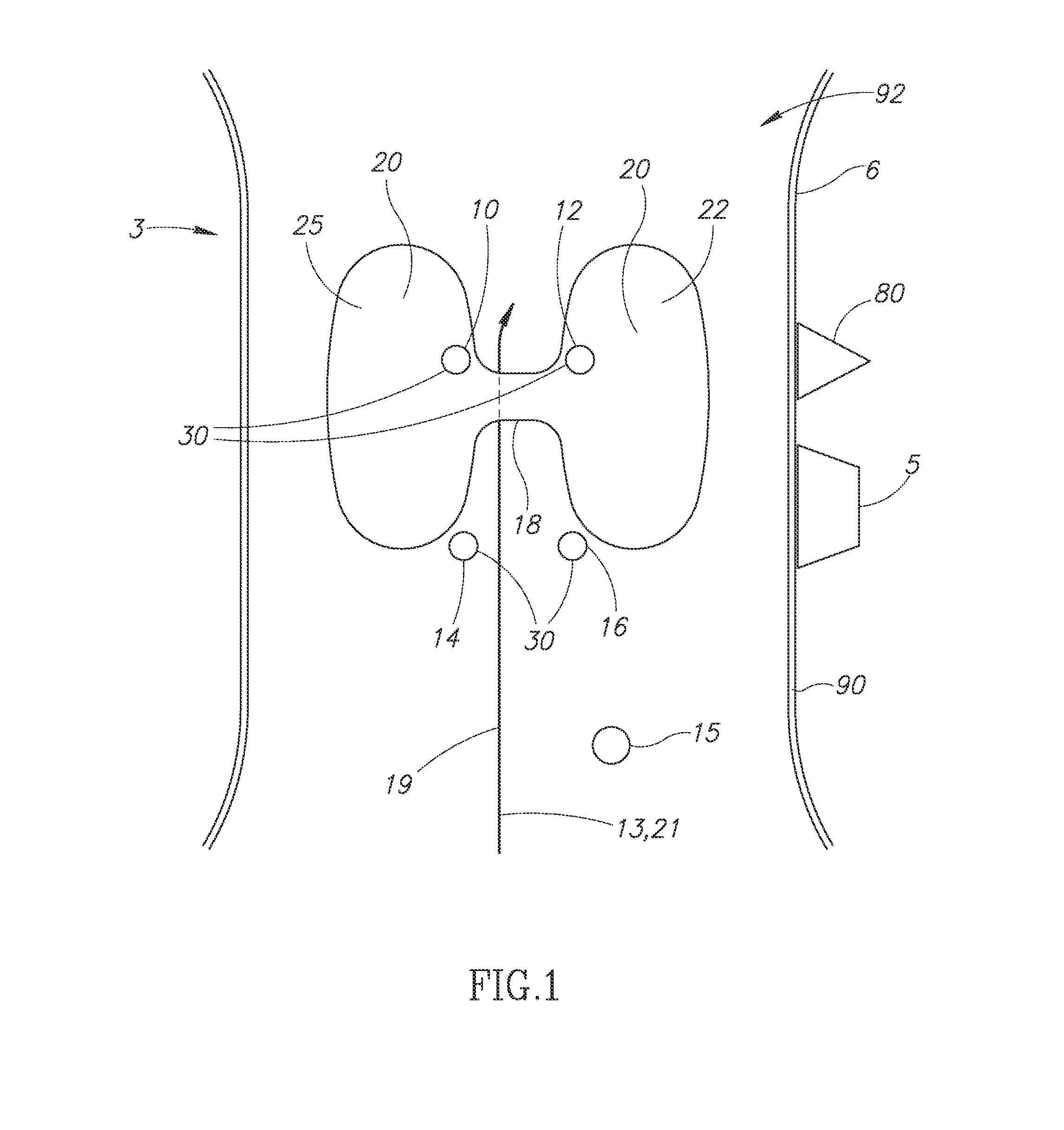
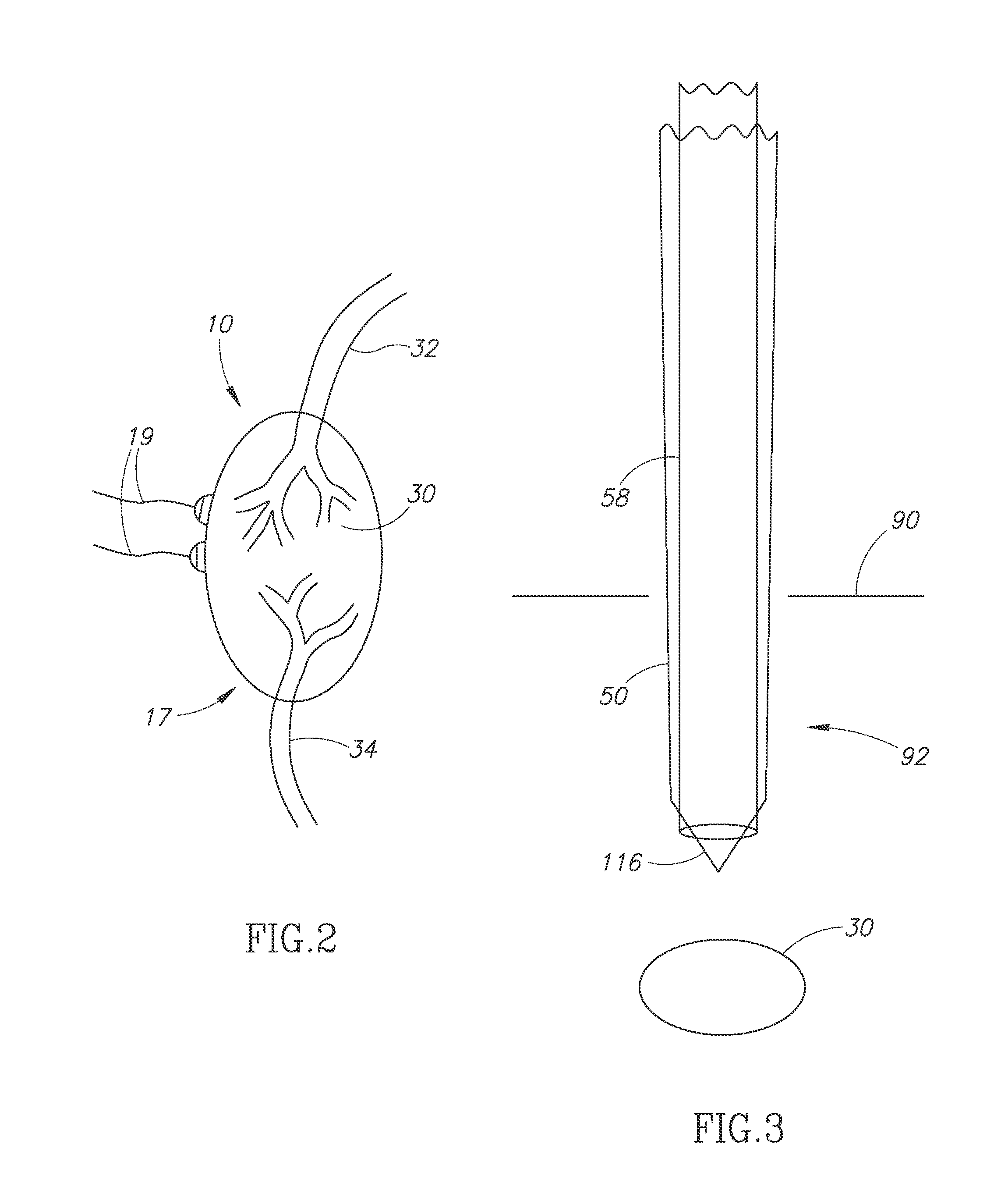
Modified delivery device for coated medical devices
InactiveUS7527632B2Minimize potential risk of damageReduce frictionStentsEar treatmentBiological bodyMedical device
Medical devices, and in particular implantable medical devices, including self-expanding stents, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
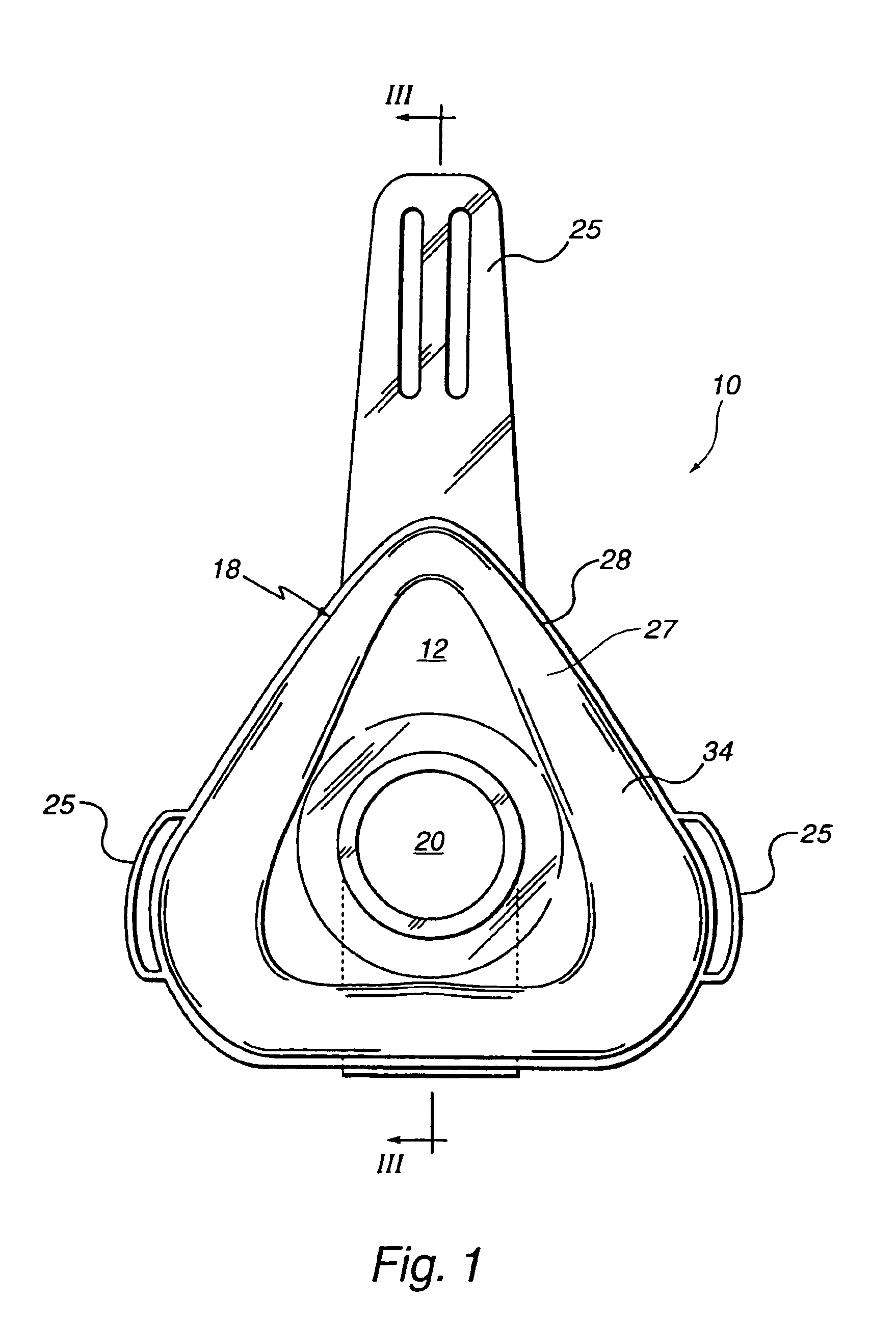
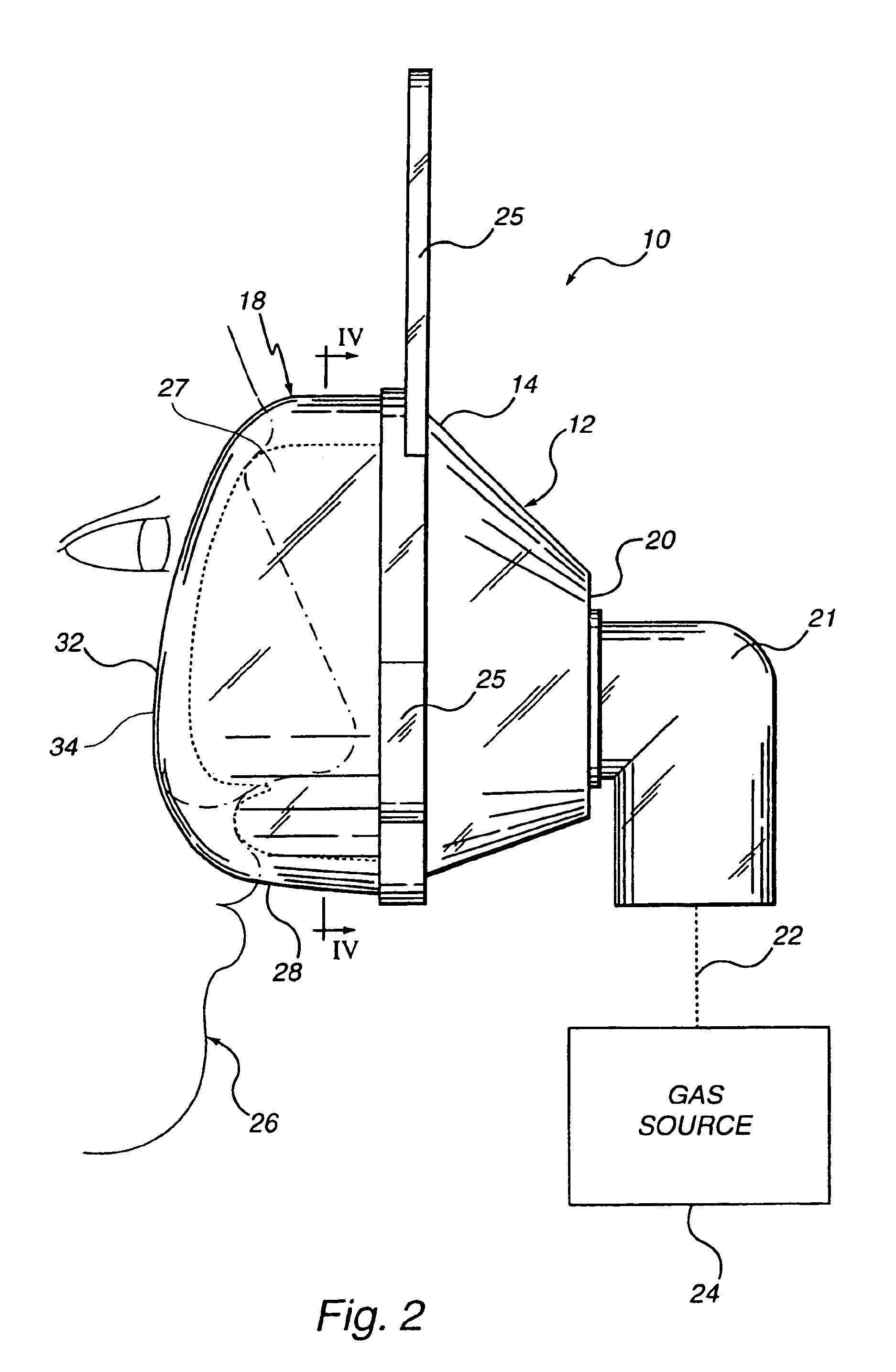
Customizable seal, mask with customizable seal and method of using such a seal
InactiveUS6895965B2Overcomes shortcomingReadily conformsRespiratory masksBreathing masksEngineeringMechanical engineering
A seal and a mask having a seal adapted for confronting engagement with a surface of a user to form an interface therewith. The seal includes a first portion defined by a gel substance and a second portion associated with the first portion. The second portion includes a selectively formable substance adapted to be molded from a first pattern into a second pattern and to retain the second pattern responsive to being so molded. The seal and mask having the seal is tailored to patient by causing the formable portion of the seal to be placed in a malleable state, applying the seal to the patient while the formable portion is in the malleable state, and causing the formable portion to be placed in a fixed state to retain a shape generally conforming to the portion of the patient underlying the seal.
Owner:RIC INVESTMENTS LLC
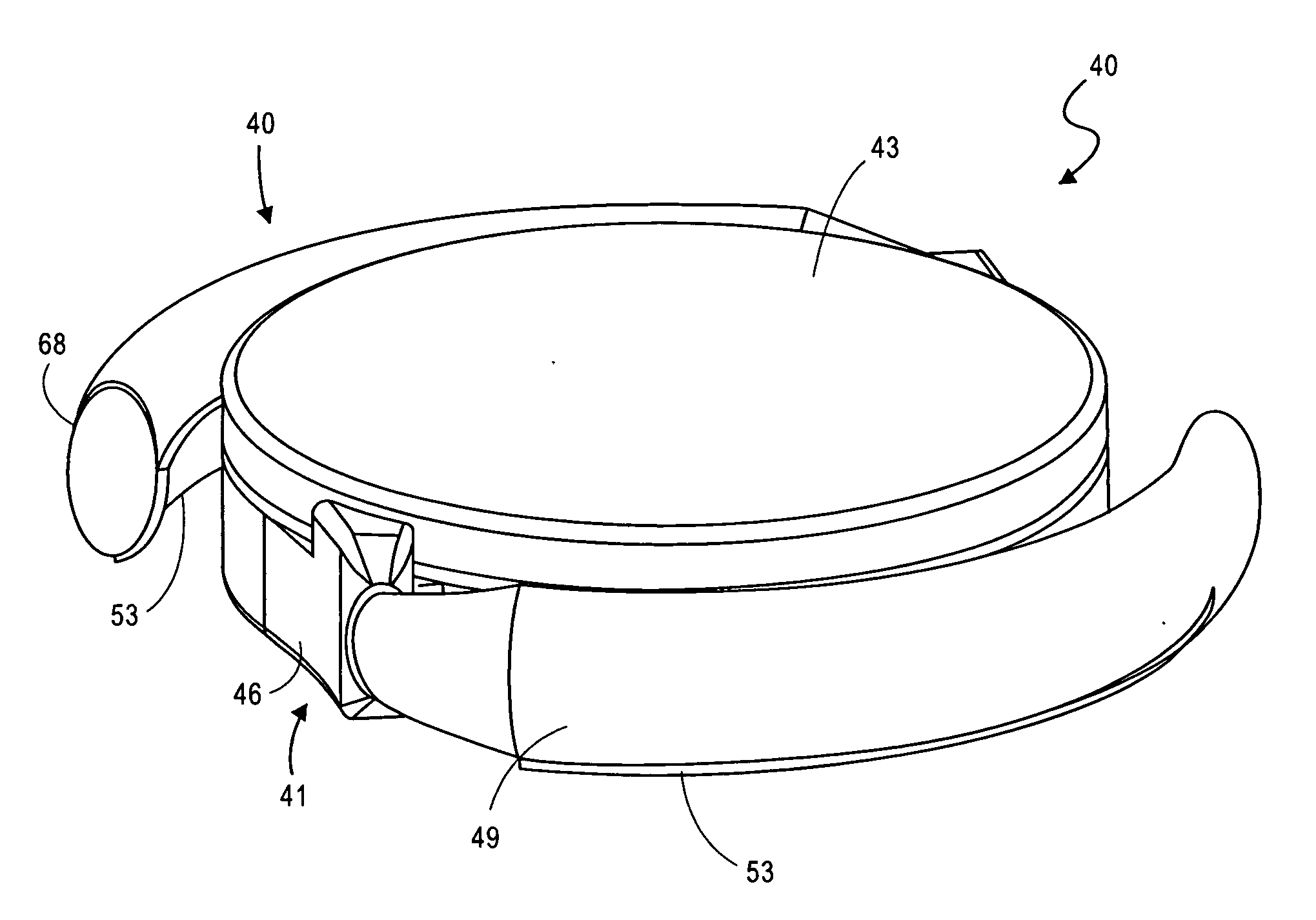
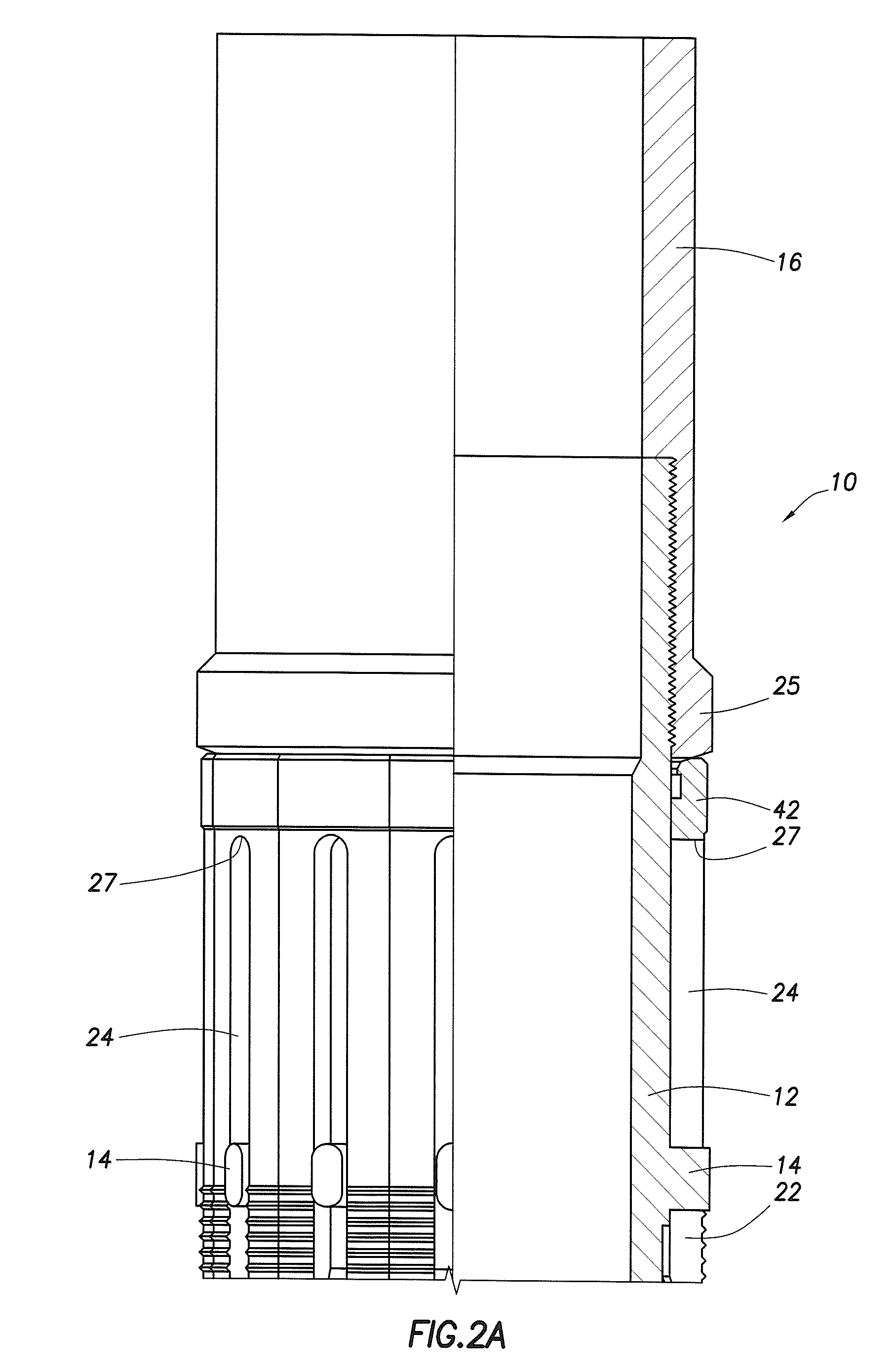
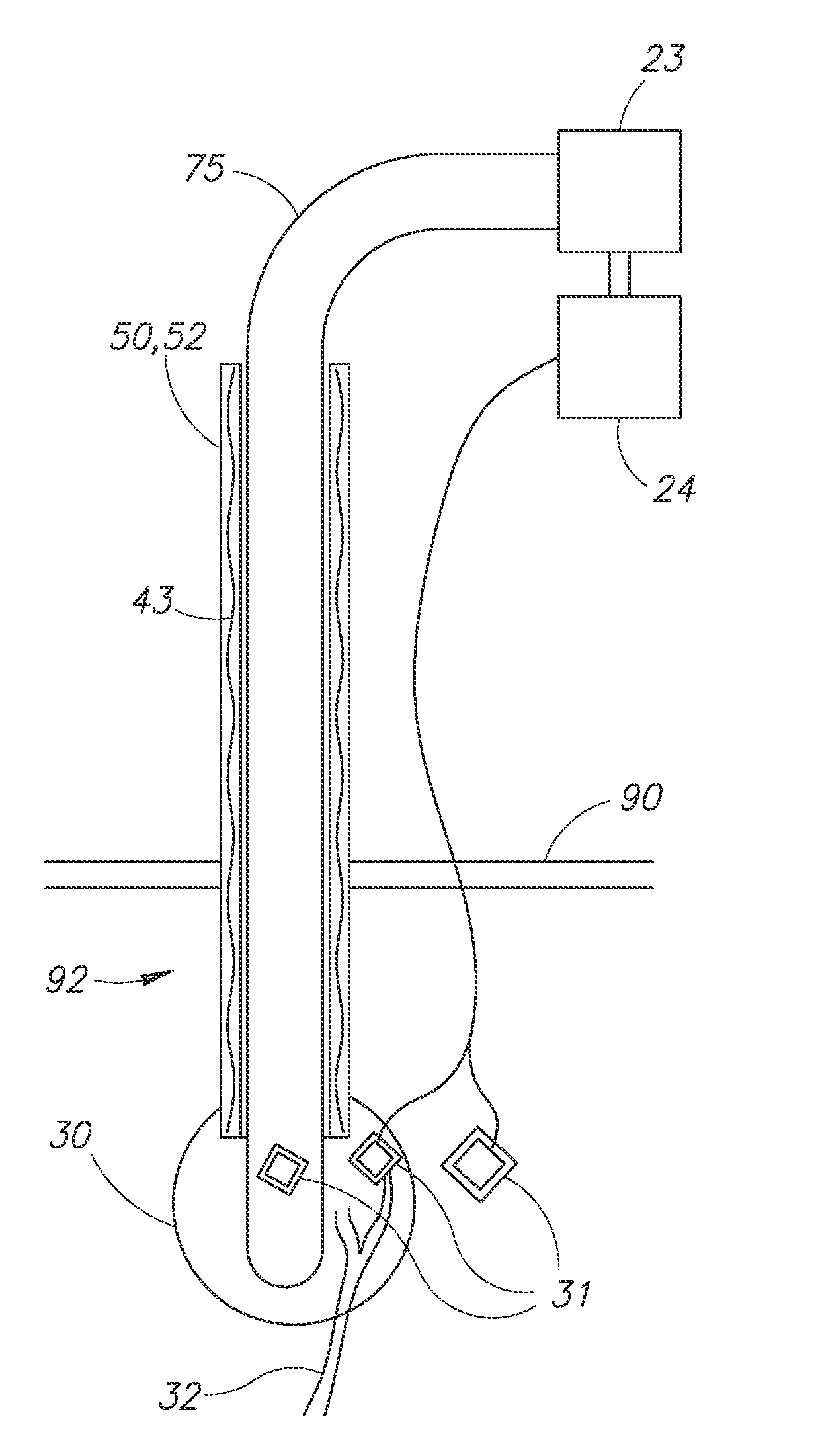
Leadless Pacemaker with Radial Fixation Mechanism
ActiveUS20120158111A1Reduce compressionTransvascular endocardial electrodesExternal electrodesDistal portionCardiac muscle
A leadless cardiac pacemaker having a radial fixation mechanism is provided. The cardiac pacemaker can include fixation mechanism separate from a pacing electrode and having a diameter equal to or less than the outer diameter of the pacemaker. The fixation mechanism can allow the pacemaker to be inserted into tissue with less than 2 rotations of the pacemaker to place the pacing electrode in contact with the tissue. In some embodiments, the fixation mechanism can comprise a plurality of hooks or protrusions positioned near a distal portion of the pacemaker. The fixation mechanism(s) can be configured to penetrate the endocardium of the patient and reside mostly within the myocardium. Methods of delivering the leadless cardiac pacemaker into the heart are also provided.
Owner:PACESETTER INC
Filter medium and structure
ActiveUS20060096263A1Efficient removalHigh strengthDispersed particle filtrationTransportation and packagingFiberParticulates
Thermoplastic bicomponent binder fiber can be combined with other media, fibers and other filtration components to form a thermally bonded filtration media. The filtration media can be used in filter units. Such filter units can be placed in the stream of a mobile fluid and can remove a particulate load from the mobile stream. The unique combination of media fiber, bicomponent binder fiber and other filtration additives and components provide a filtration media having unique properties in filtration applications.
Owner:DONALDSON CO INC
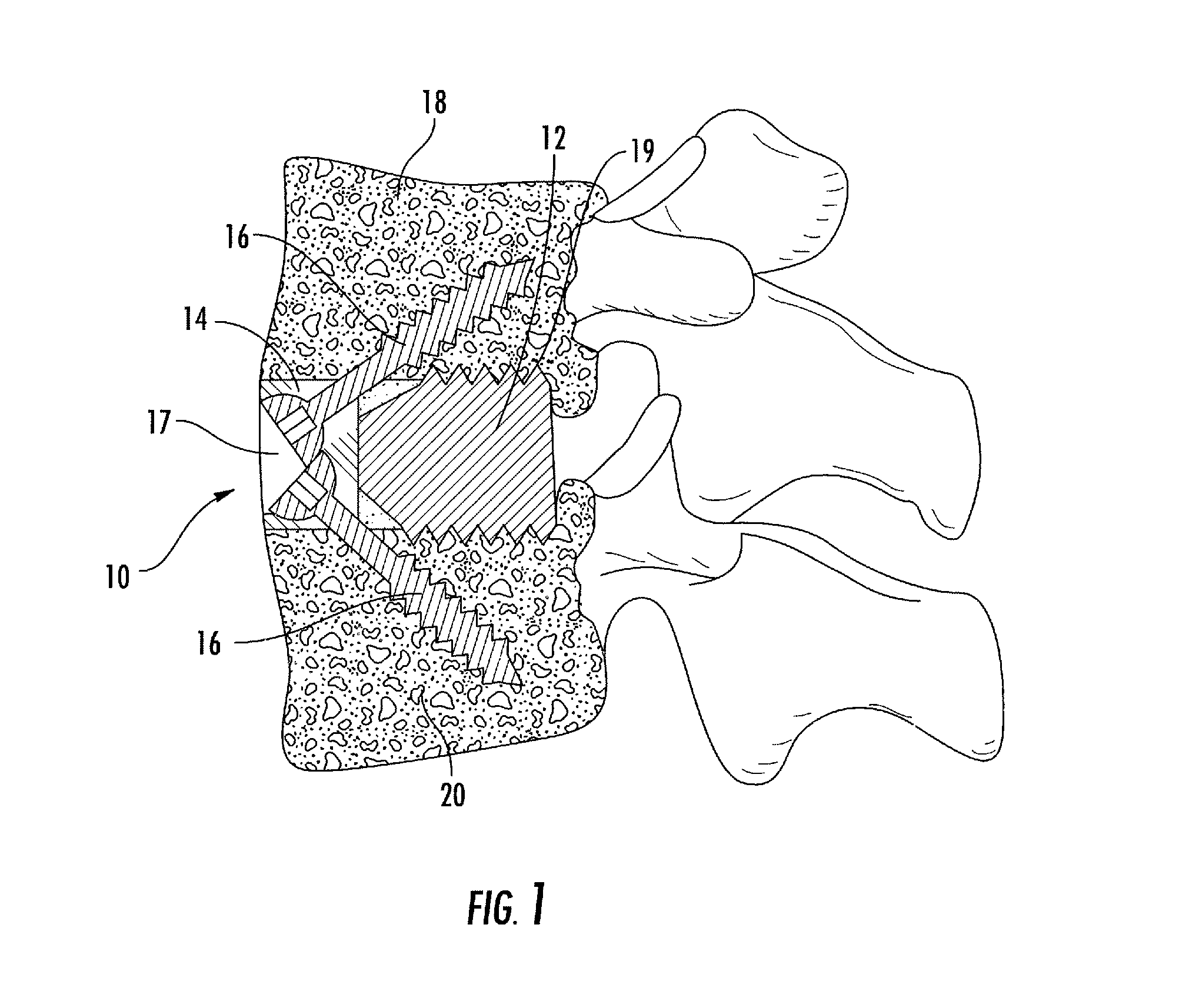
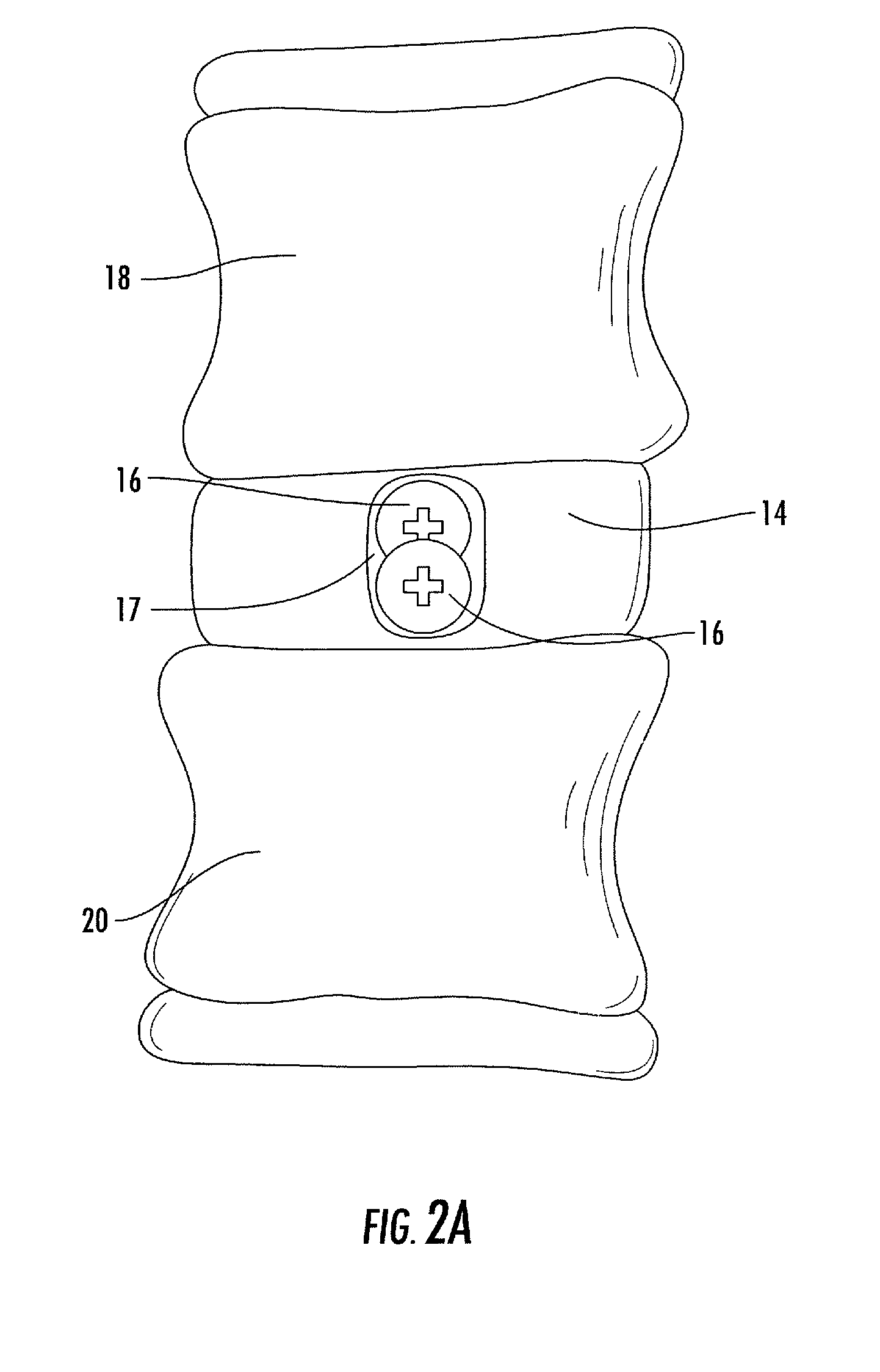
Interbody fusion system with intervertebral implant retention assembly
ActiveUS20100217393A1Eliminating exacerbation of instabilityImprove stabilitySpinal implantsFastenersPosterior instrumentationCorpectomy
The present disclosure is directed towards a biomechanical implant and anterior, lateral or posterior instrumentation construct. The construct may be of unitary or modular construction, whereby a single molded construction can form the entire assembly, in which case the through holes may be adapted to receive a metallic insert for screw fixation; or alternatively be of a modular construction wherein the anterior / lateral instrumentation and intervertebral spacer are designed for removable locking engagement, one with the other, for insertion by the surgeon as a unitary construct. A unique feature of the construct resides within the instrumentation construction, whereby a single opening formed therein permits two bone screws, or the like fastener device, to be positioned within both the superior and inferior vertebral bodies surrounding the spacer implant, or, for example in the case of a corpectomy or diskectomy with cage insertion, wherein two screws can be fixed within a single vertebral body through a single through hole, and wherein the bone screws are constructed and arranged to cooperate with the retention plate so as to provide locking engagement, one to the other, with the retention plate, upon final fixation thereof. Screw retention elements of alternative shape, based upon the choice of vertical or horizontal orientation, based upon an opened figure eight design, are provided for insertion in a groove formed in the borehole of the instrumentation plate which allows insertion of each fixation element but will prevent a loosened fixation element from falling out of the plate.
Owner:SPARTAN CAGE HLDG
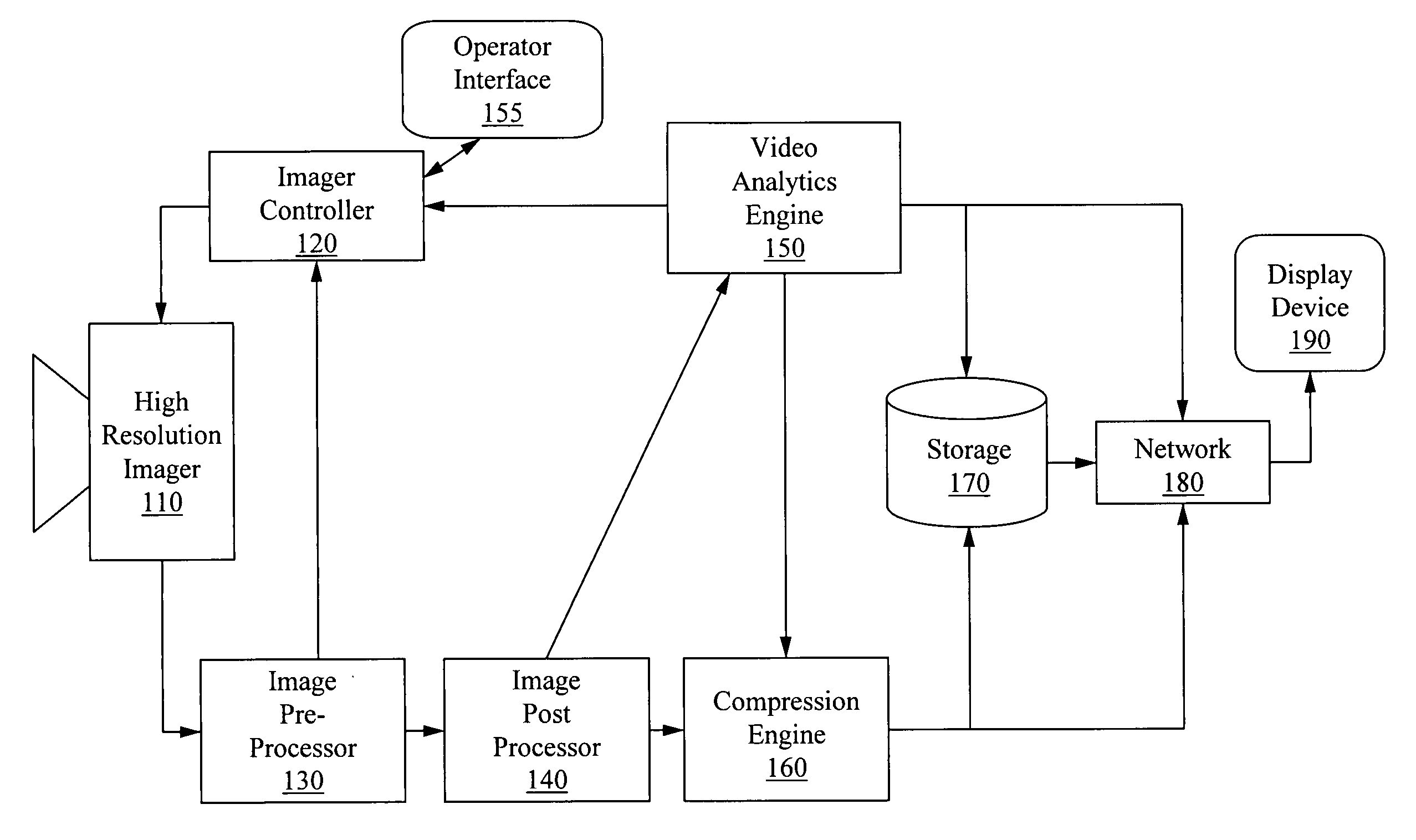
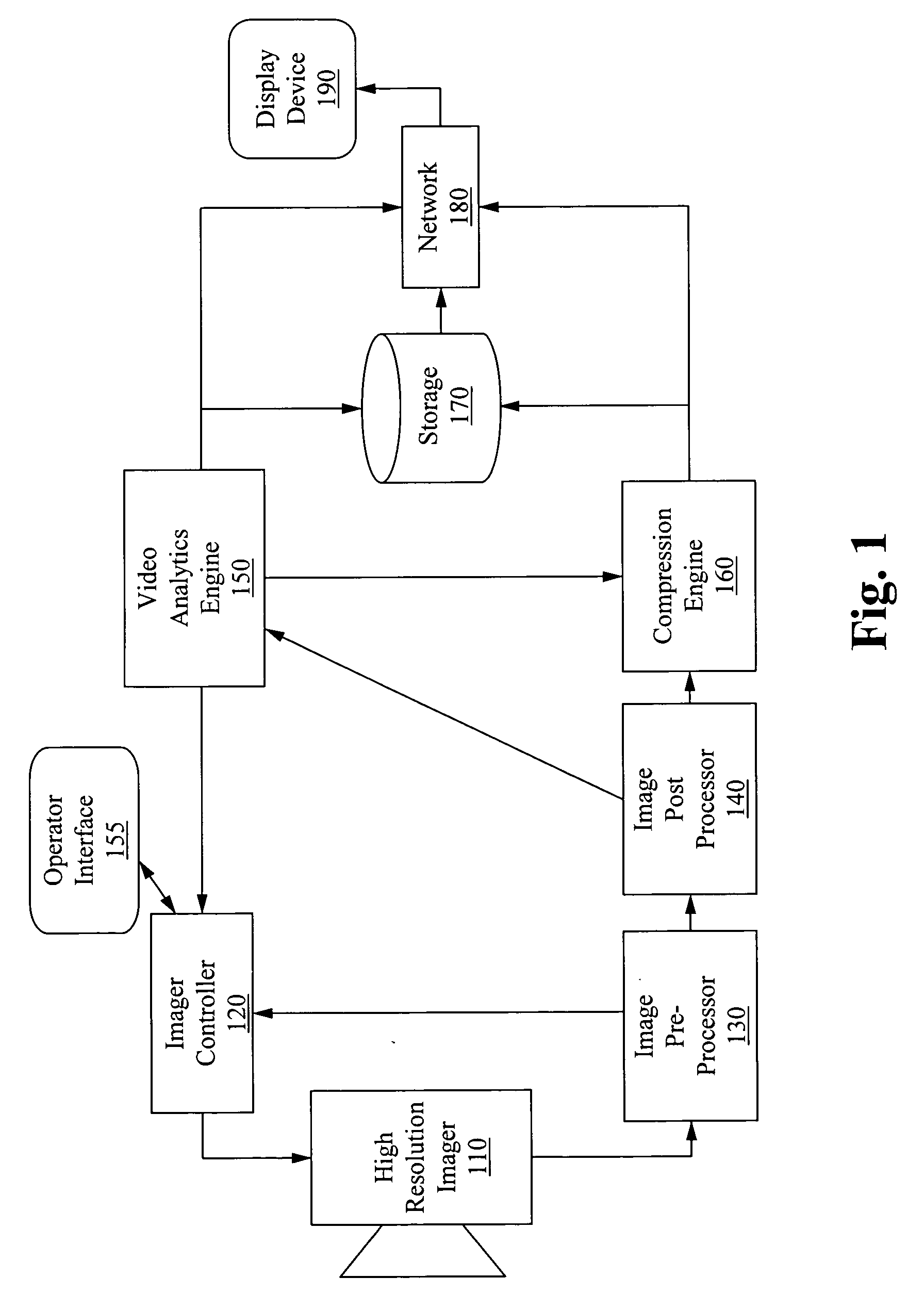
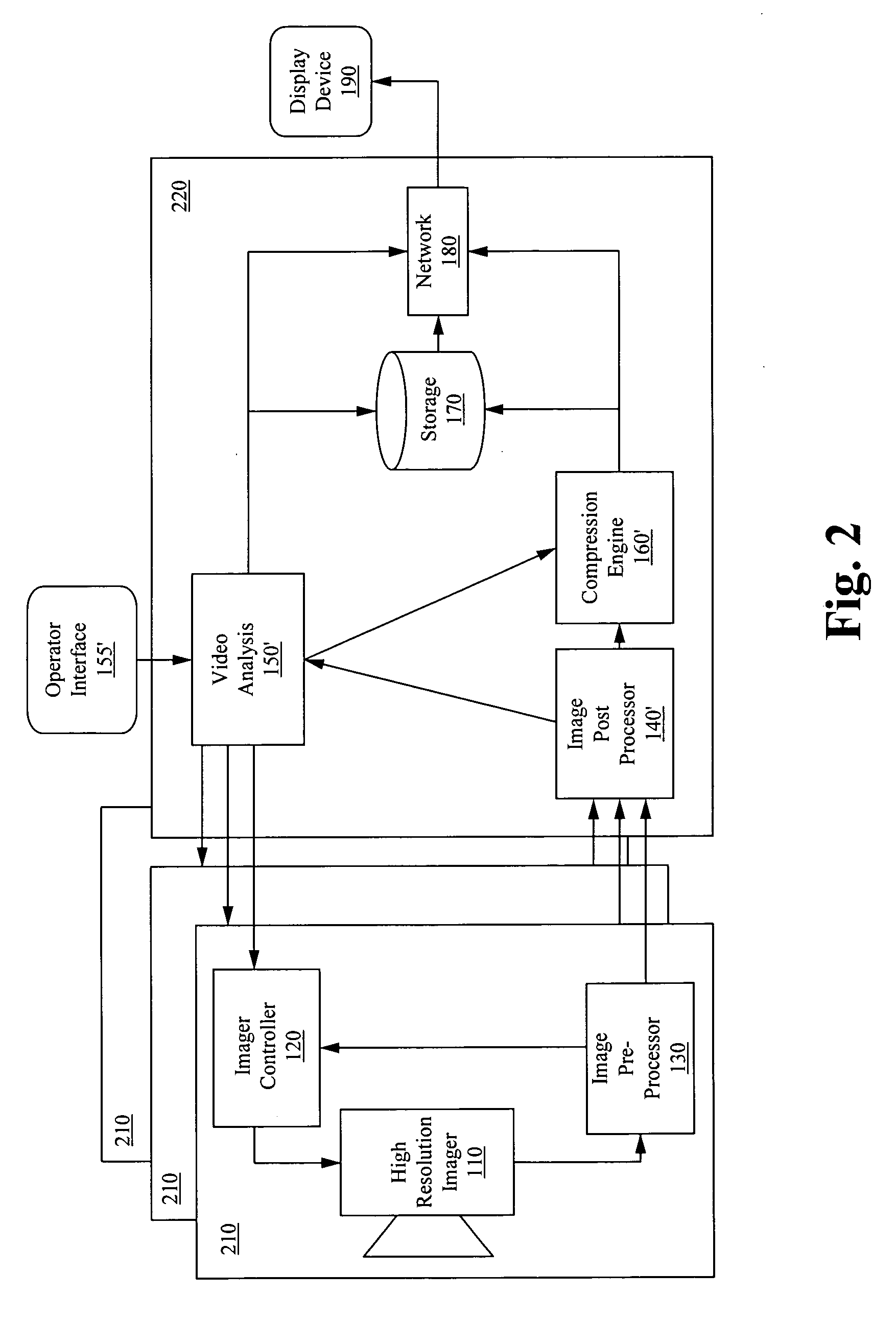
Apparatus for image capture with automatic and manual field of interest processing with a multi-resolution camera
InactiveUS20080129844A1Resolution and frame rate and clarityHigh compression levelTelevision system detailsColor television detailsRegion of interestDistinctness of image
An apparatus for capturing a video image comprising a means for generating a digital video image, a means for classifying the digital video image into one or more regions of interest and a background image, and a means for encoding the digital video image, wherein the encoding is selected to provide at least one of; enhancement of the image clarity of the one or more ROI relative to the background image encoding, and decreasing the video quality of the background image relative to the one or more ROI. A feedback loop is formed by the means for classifying the digital video image using a previous video image to generate a new ROI and thus allow for tracking of targets as they move through the imager field-of-view.
Owner:AGILENCE
Filter medium and breather filter structure
Thermoplastic bicomponent binder fiber can be combined with other media, fibers and other filtration components to form a thermally bonded filtration media. The filtration media can be used in filter units, such as breather caps. Such filter units can be placed in the stream of a mobile fluid and can remove a particulate and / or fluid mist load from the mobile stream. The unique combination of media fiber, bicomponent binder fiber and other filtration additives and components provide a filtration media having unique properties in filtration applications.
Owner:DONALDSON CO INC
Filter element and method
InactiveUS20090044702A1Life of element can be lengthenedIncrease capacityCombination devicesNon-fibrous pulp additionMultiple formsParticulates
A filter element having multiple formed layers of filtration media is disclosed. The media are layered so as to form a pore size gradient. The filter element is capable of removing both solid and liquid particulates from a moving fluid stream. The filter element has high strength and compressibility. The layers can be supported on a porous or perforate support to provide mechanical stability during filtering operations. The filtration media layers can be formed into various filter element forms such as panels, cartridges, inserts, and the like.
Owner:DONALDSON CO INC
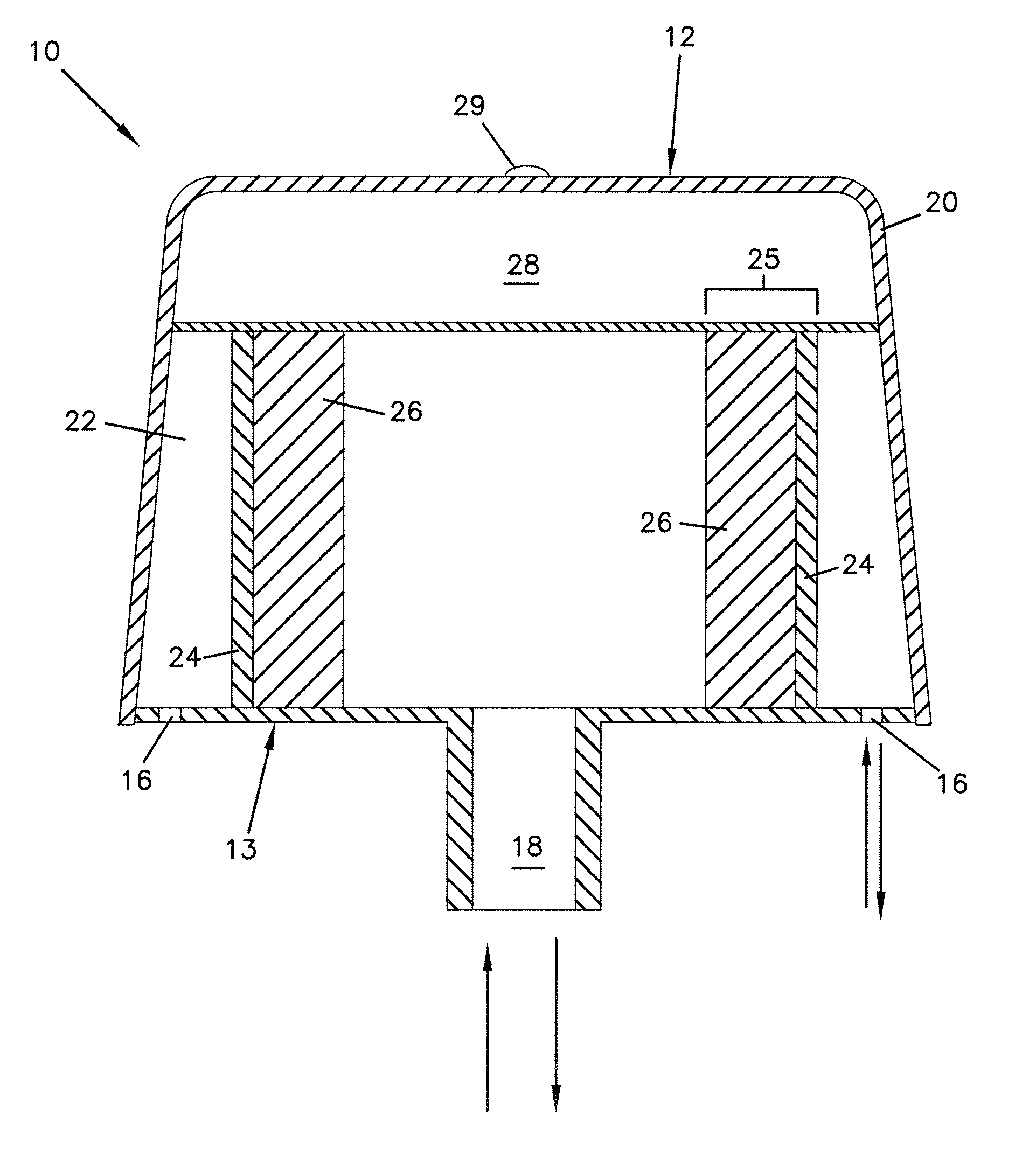
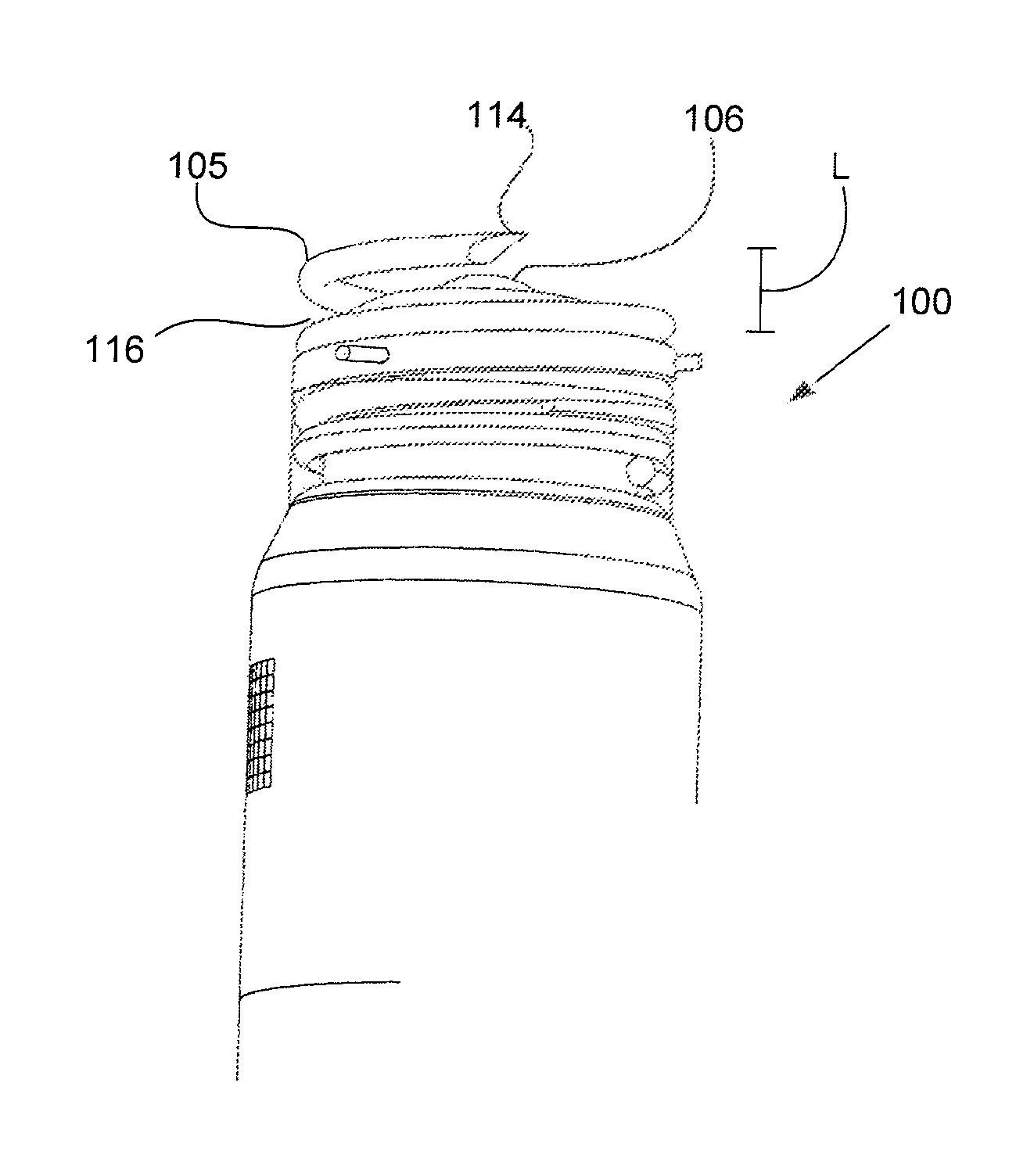
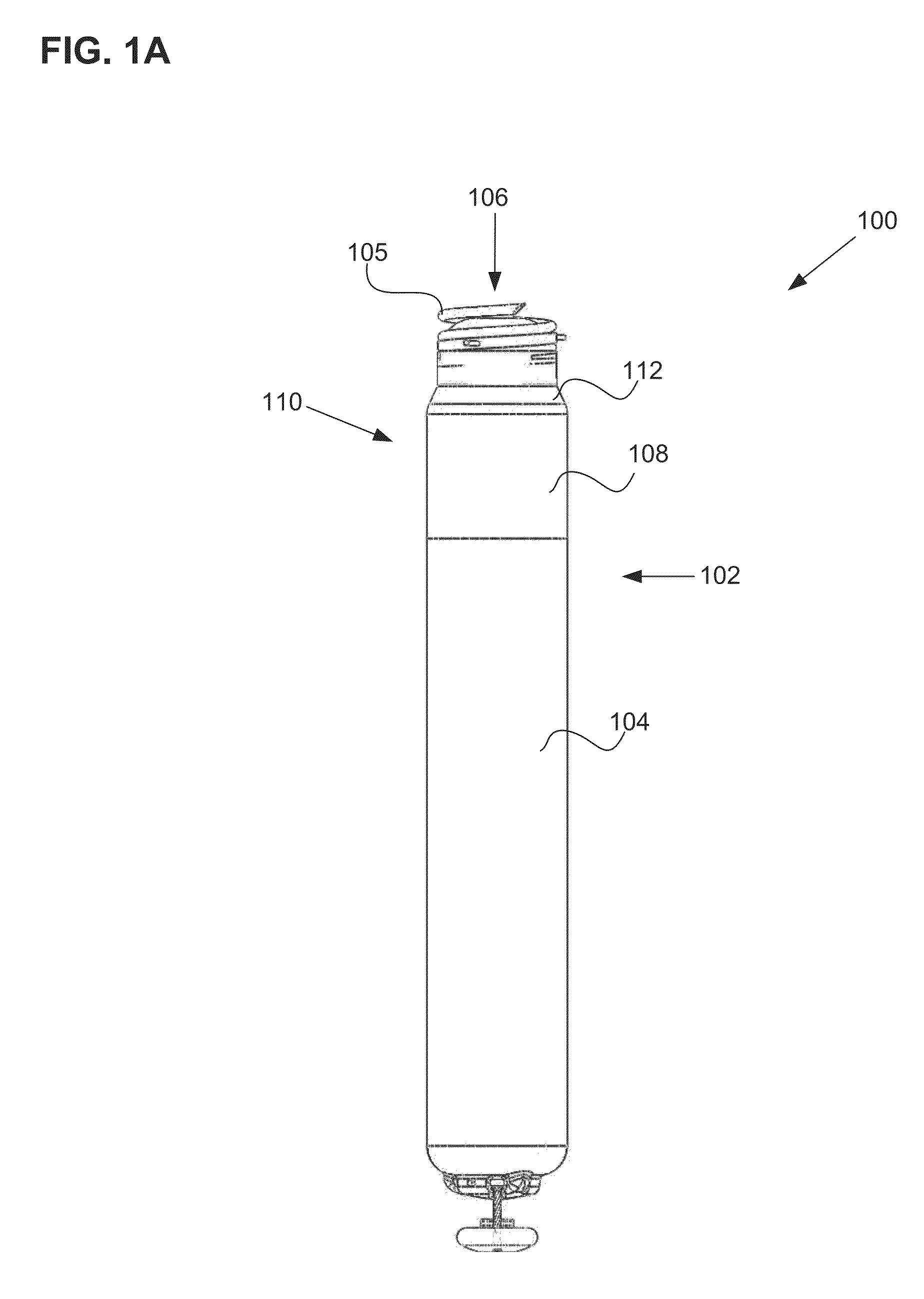
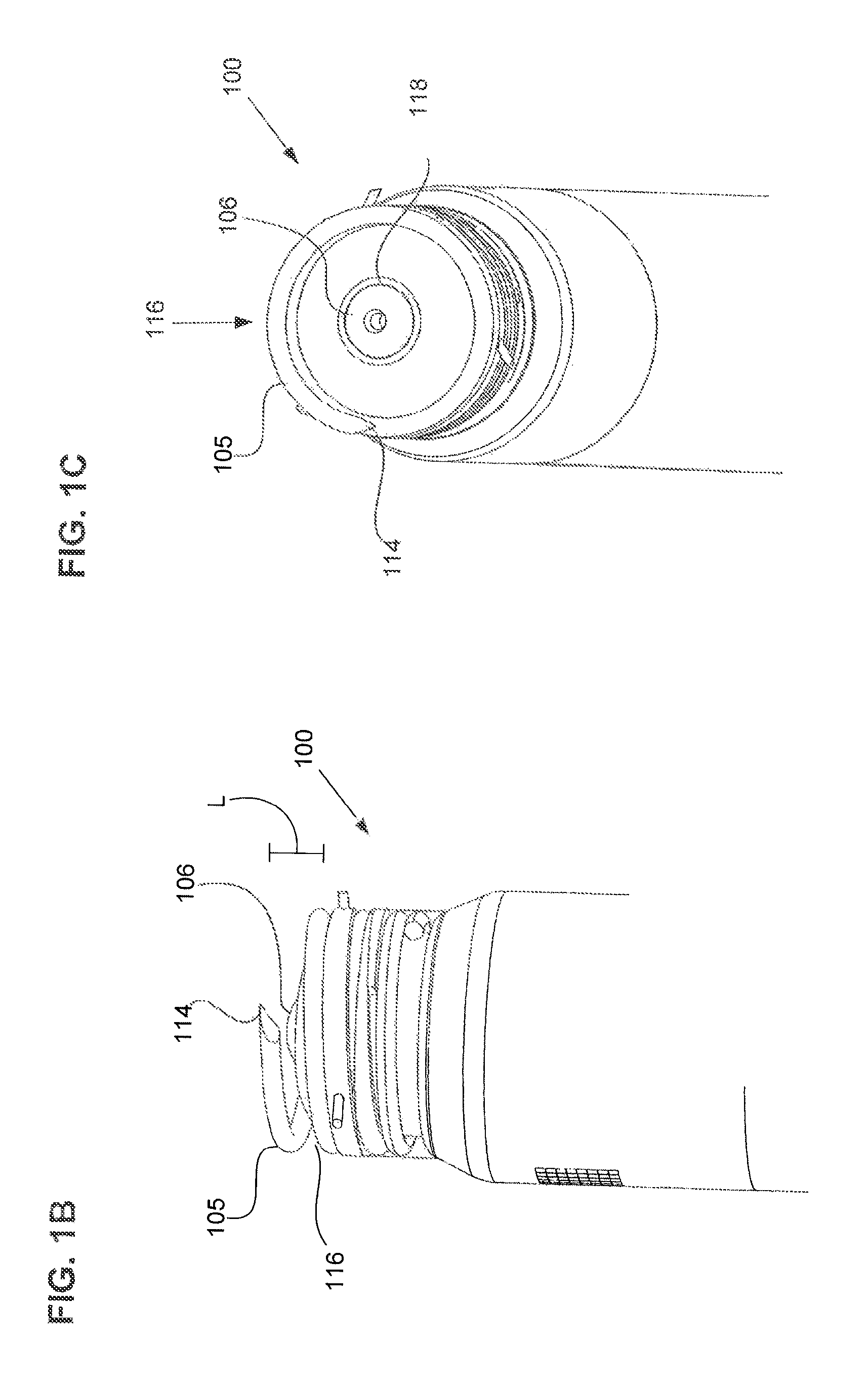
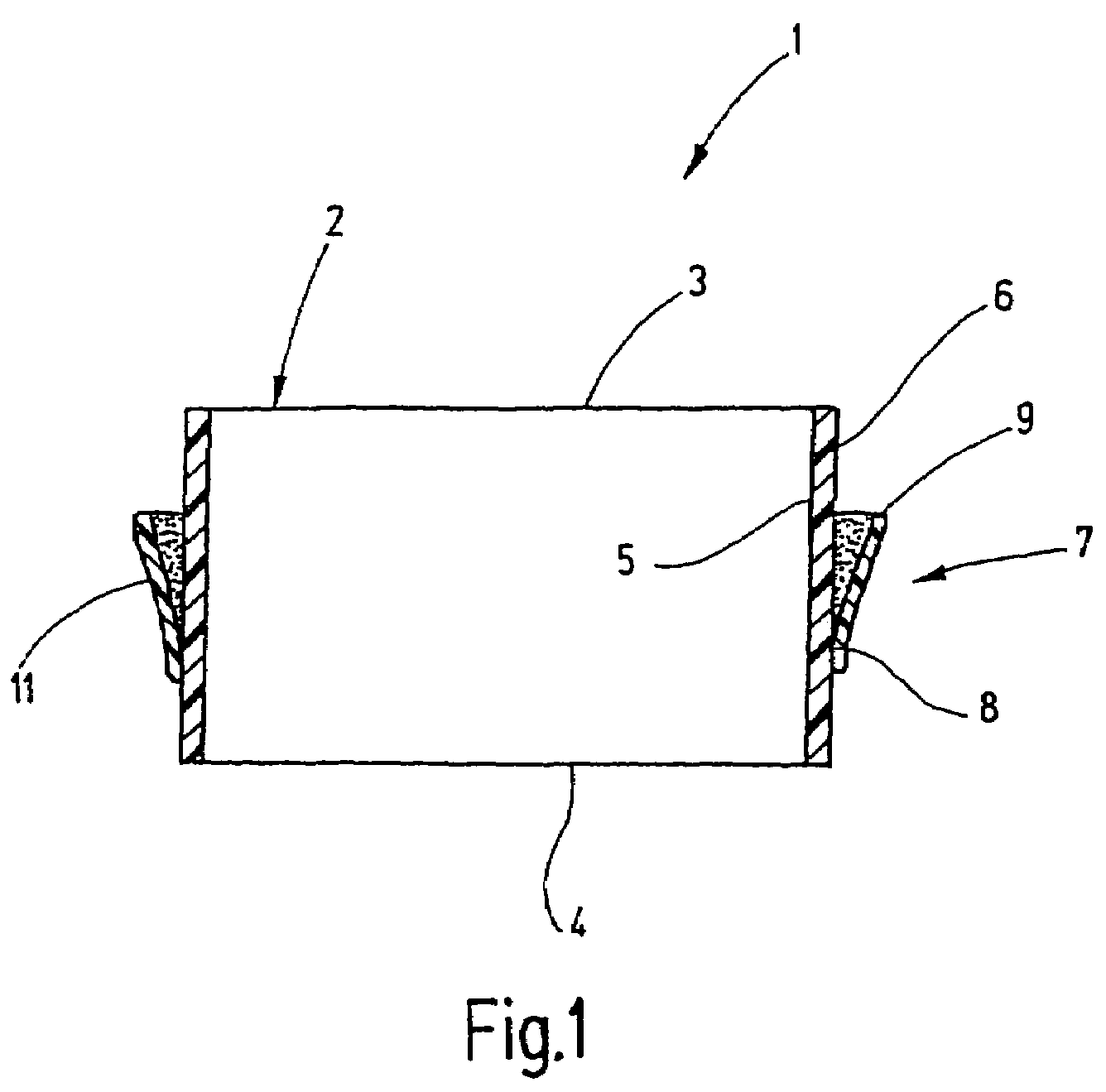
Synthetic resin container closure
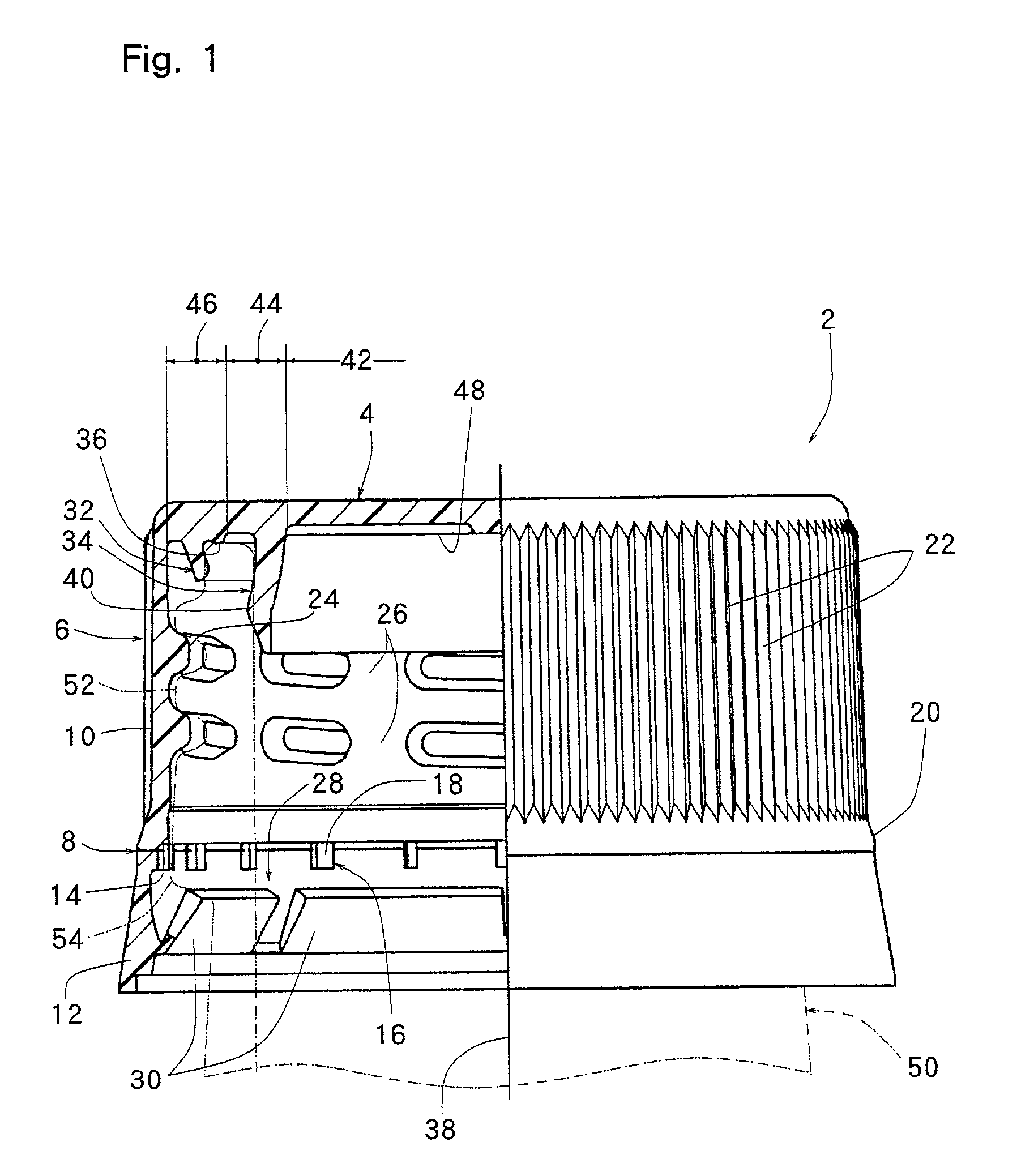
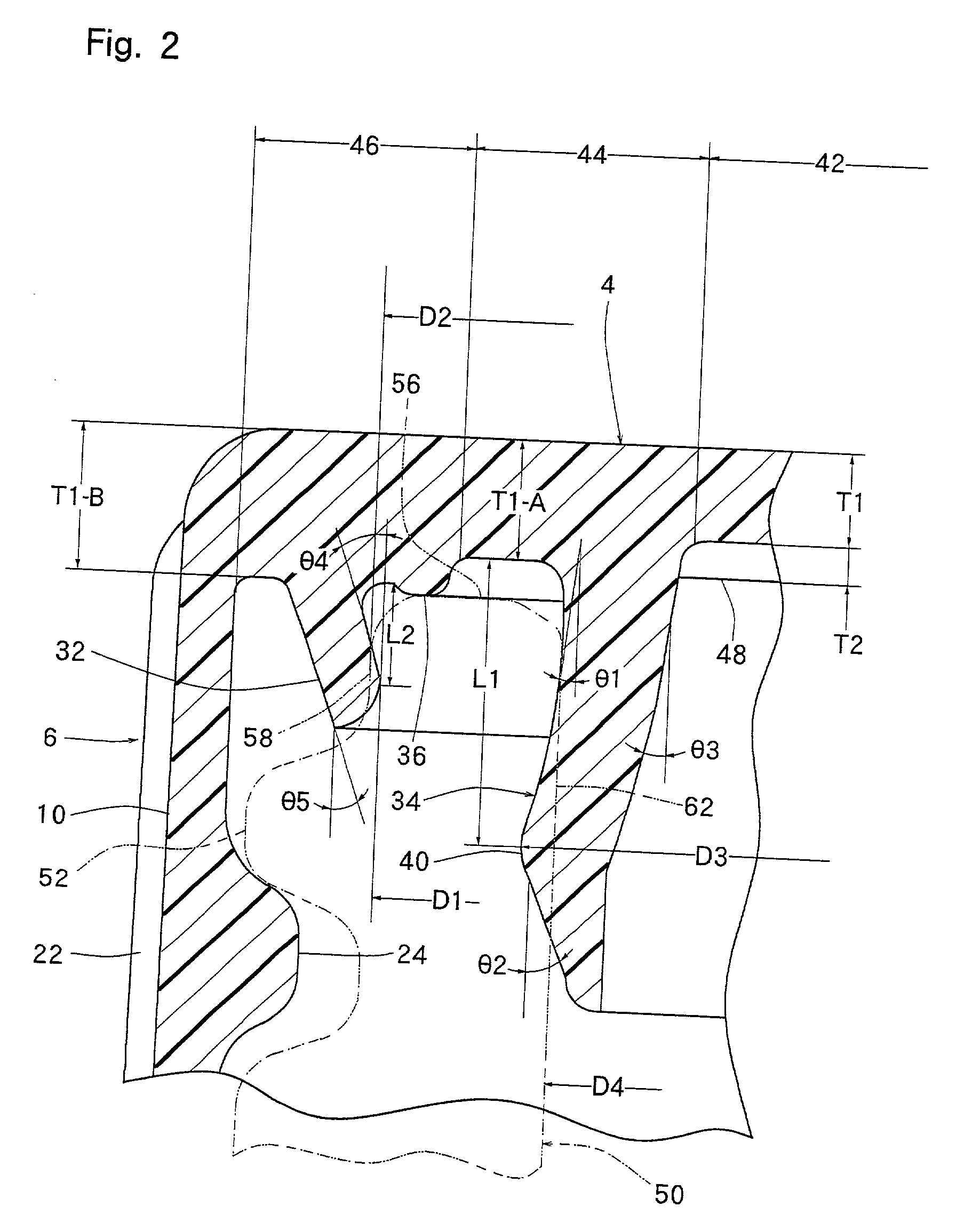
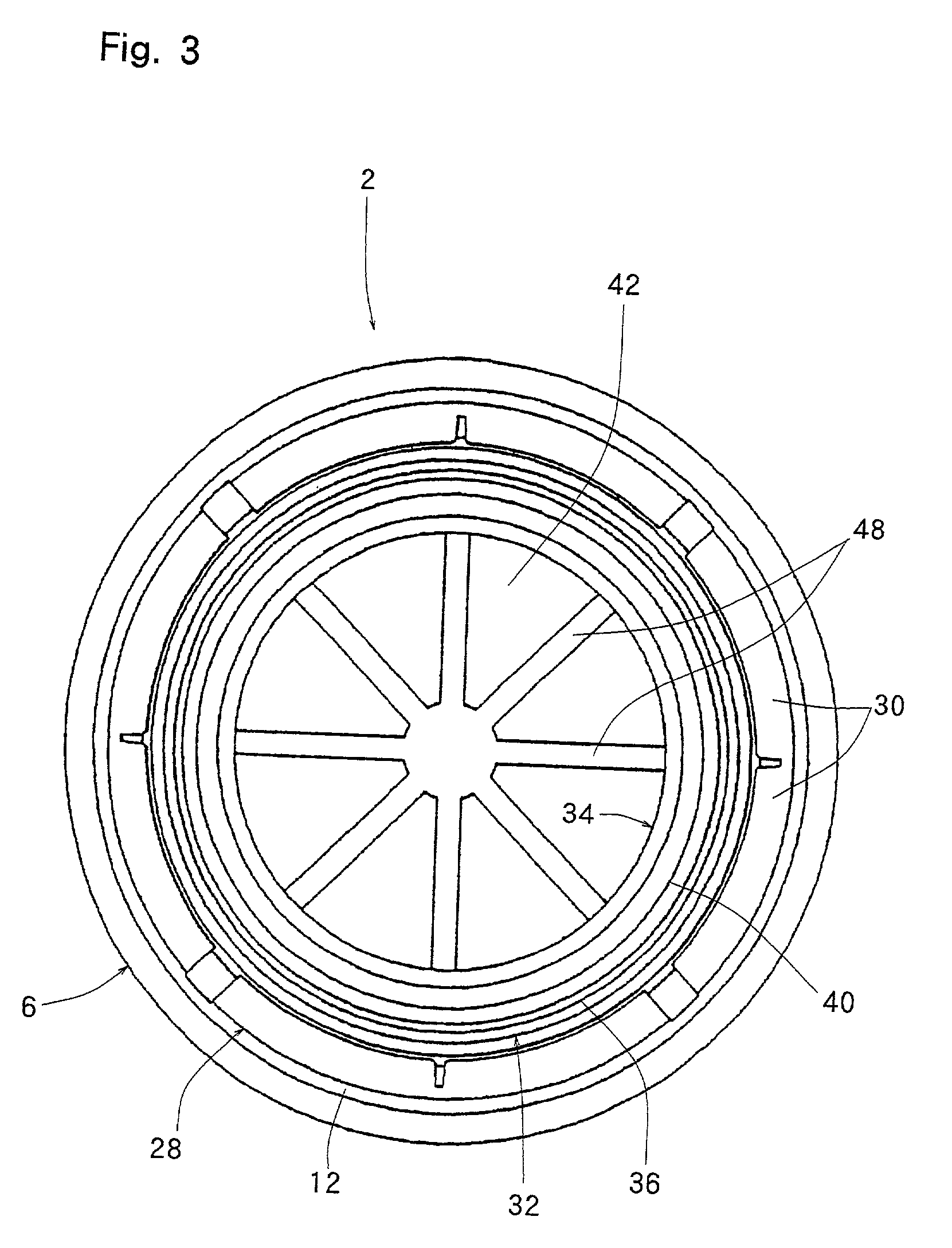
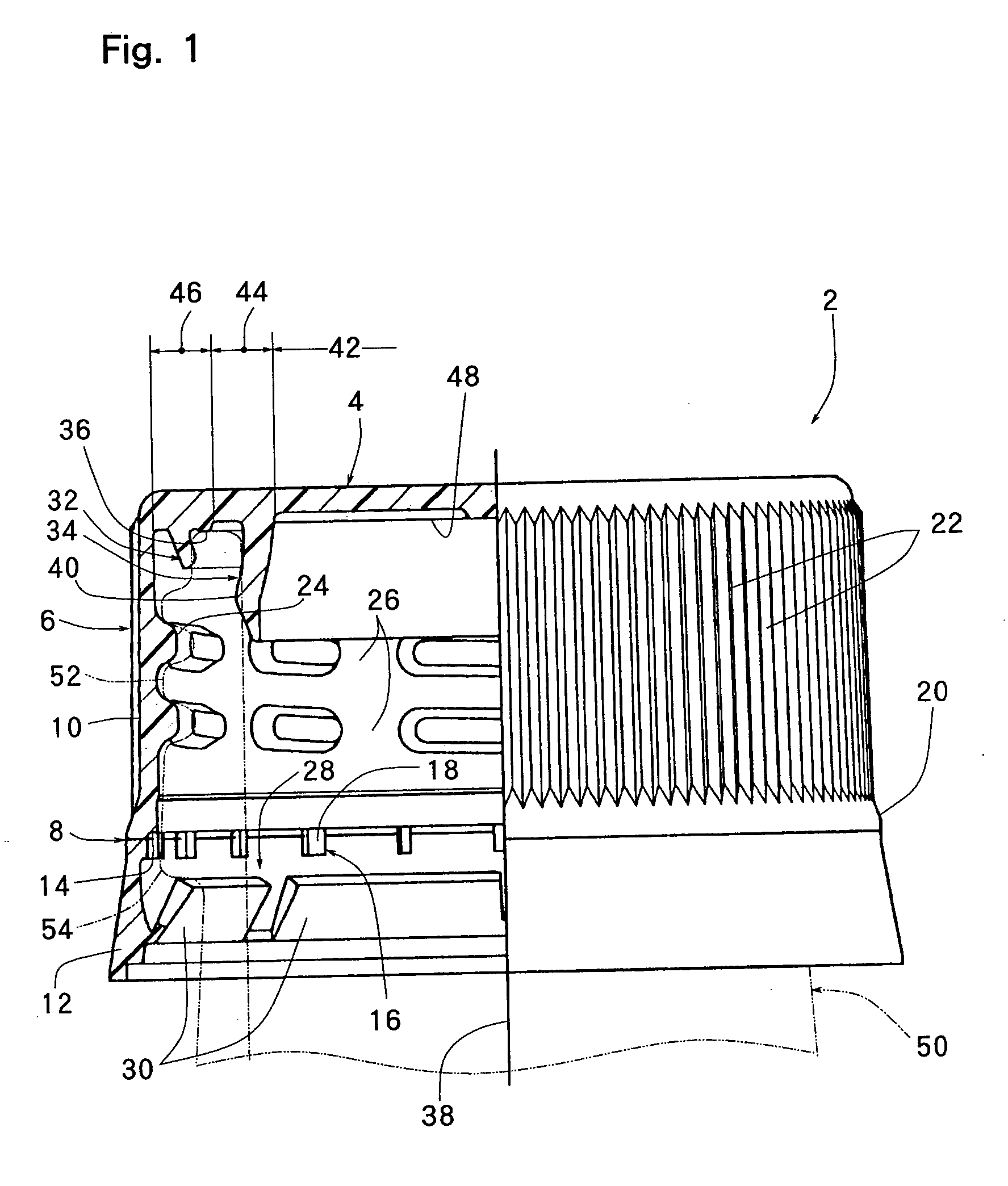
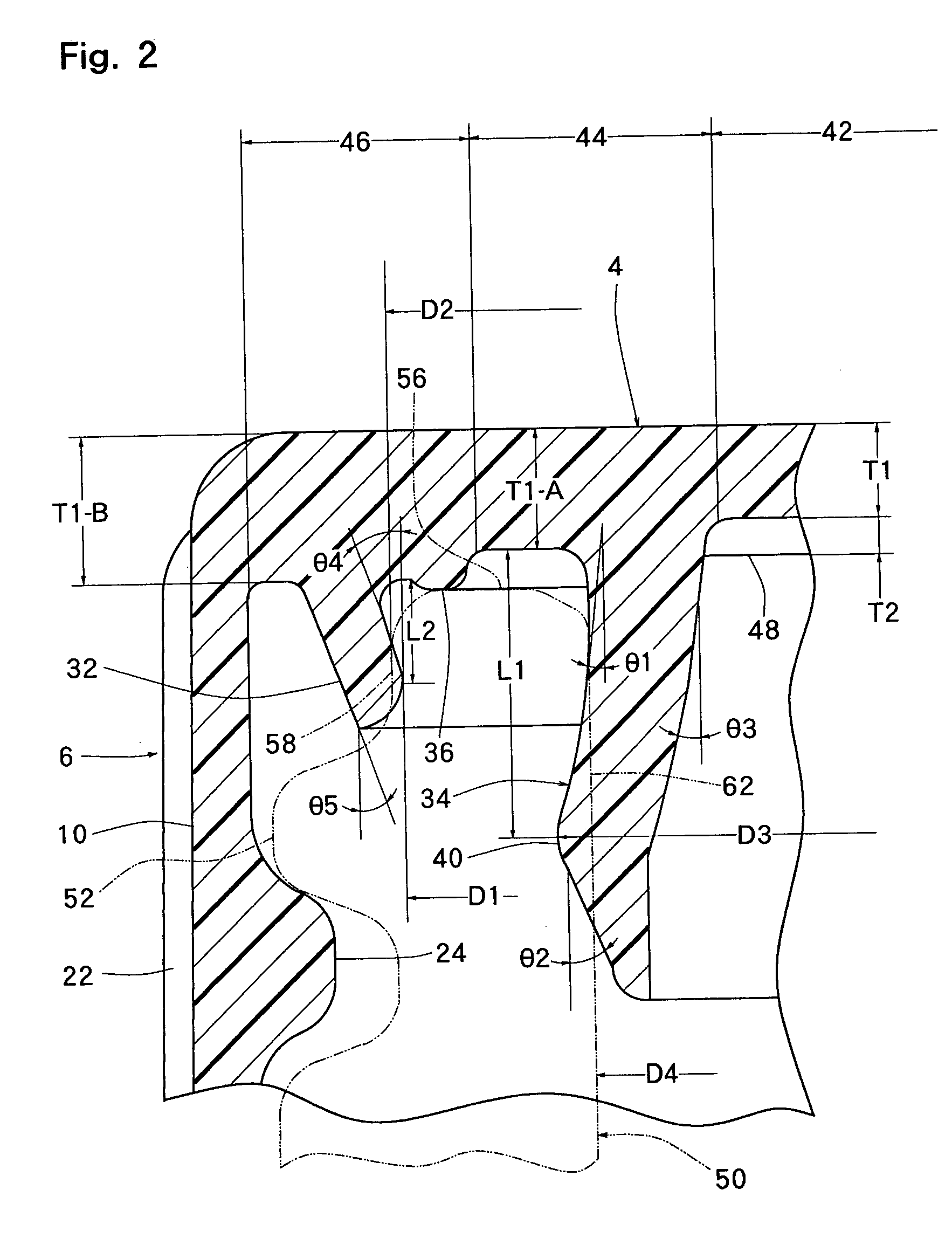
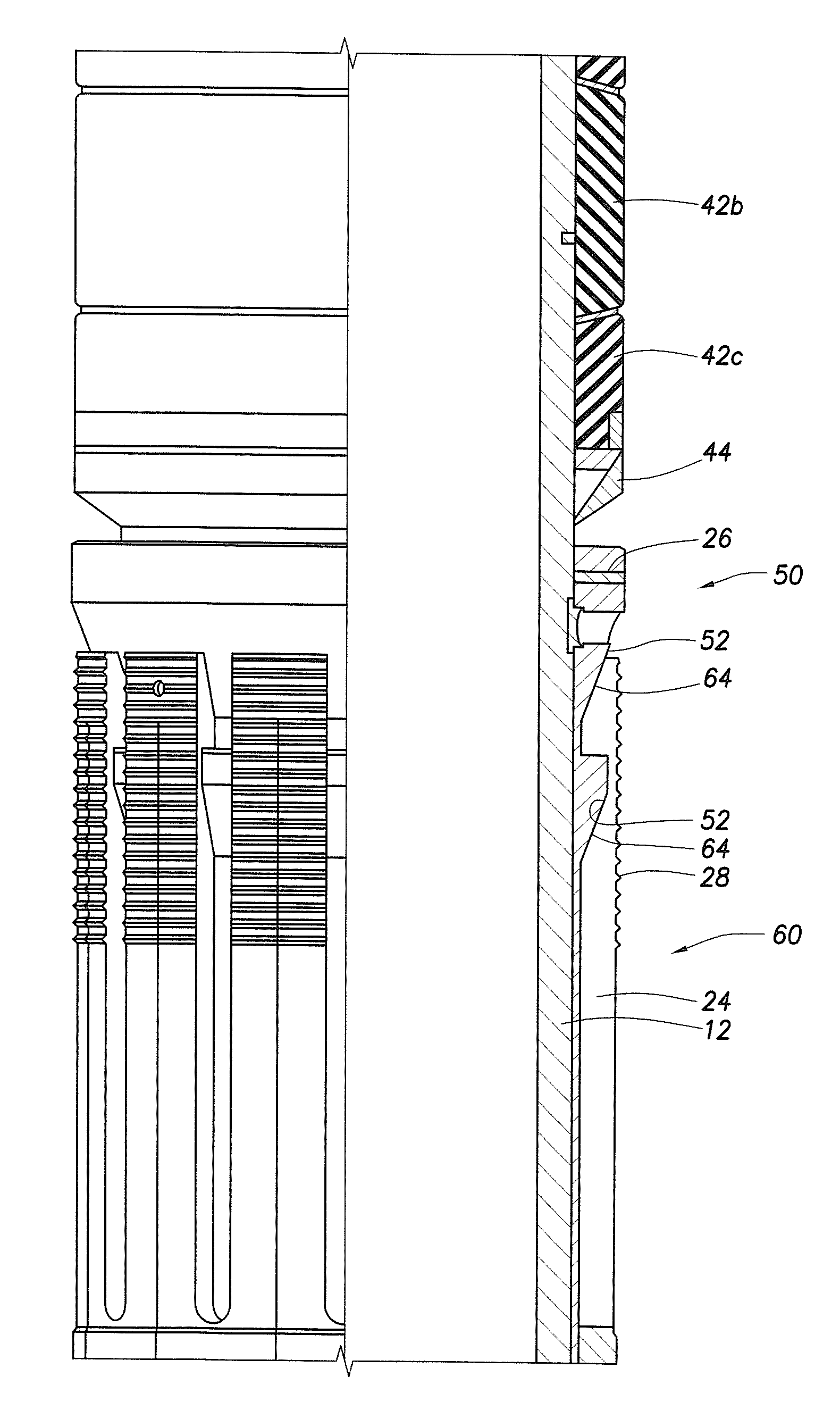
InactiveUS20010027957A1Reduce compressionReduce injectionCapsClosure using stoppersEngineeringMechanical engineering
A container closure formed from a synthetic resin as a single unit has a circular top panel wall and a cylindrical skirt wall extending downwardly from the peripheral edge of the top panel wall. An outer cylindrical sealing protrusion, inner cylindrical sealing protrusion and annular sealing ridge arranged therebetween, all having a predetermined shape and a predetermined size, are formed on the inner surface of the top panel wall. The thickness of the center portion of the top panel wall is reduced to a predetermined range and a plurality of ribs having a predetermined thickness are formed on the inner surface of the center portion of the top panel wall.
Owner:JAPAN CROWN CORK CO LTD
Leadless pacemaker with radial fixation mechanism
ActiveUS9242102B2Reduce compressionTransvascular endocardial electrodesExternal electrodesDistal portionCardiac muscle
A leadless cardiac pacemaker having a radial fixation mechanism is provided. The cardiac pacemaker can include fixation mechanism separate from a pacing electrode and having a diameter equal to or less than the outer diameter of the pacemaker. The fixation mechanism can allow the pacemaker to be inserted into tissue with less than 2 rotations of the pacemaker to place the pacing electrode in contact with the tissue. In some embodiments, the fixation mechanism can comprise a plurality of hooks or protrusions positioned near a distal portion of the pacemaker. The fixation mechanism(s) can be configured to penetrate the endocardium of the patient and reside mostly within the myocardium. Methods of delivering the leadless cardiac pacemaker into the heart are also provided.
Owner:PACESETTER INC
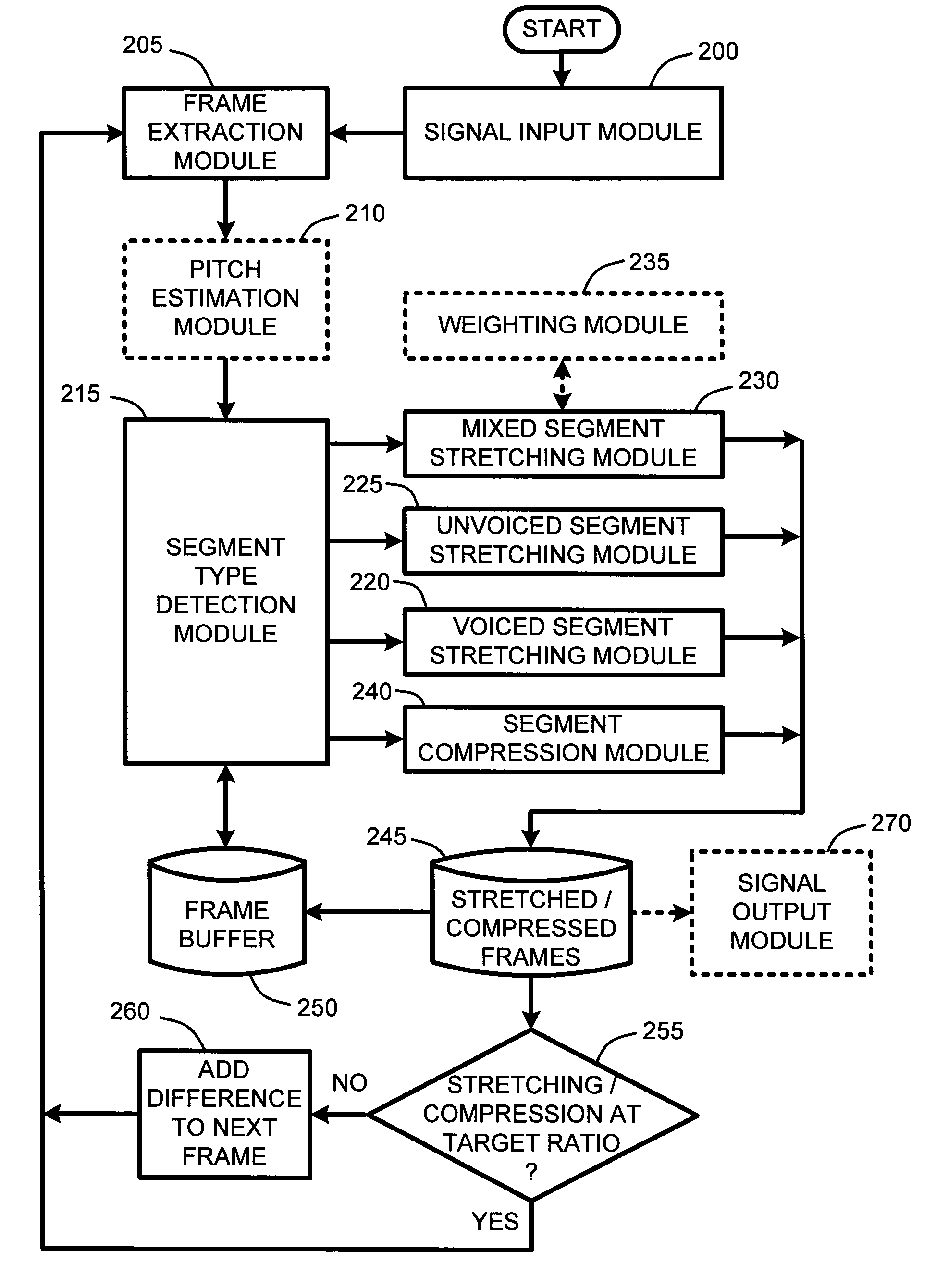
System and method for providing high-quality stretching and compression of a digital audio signal
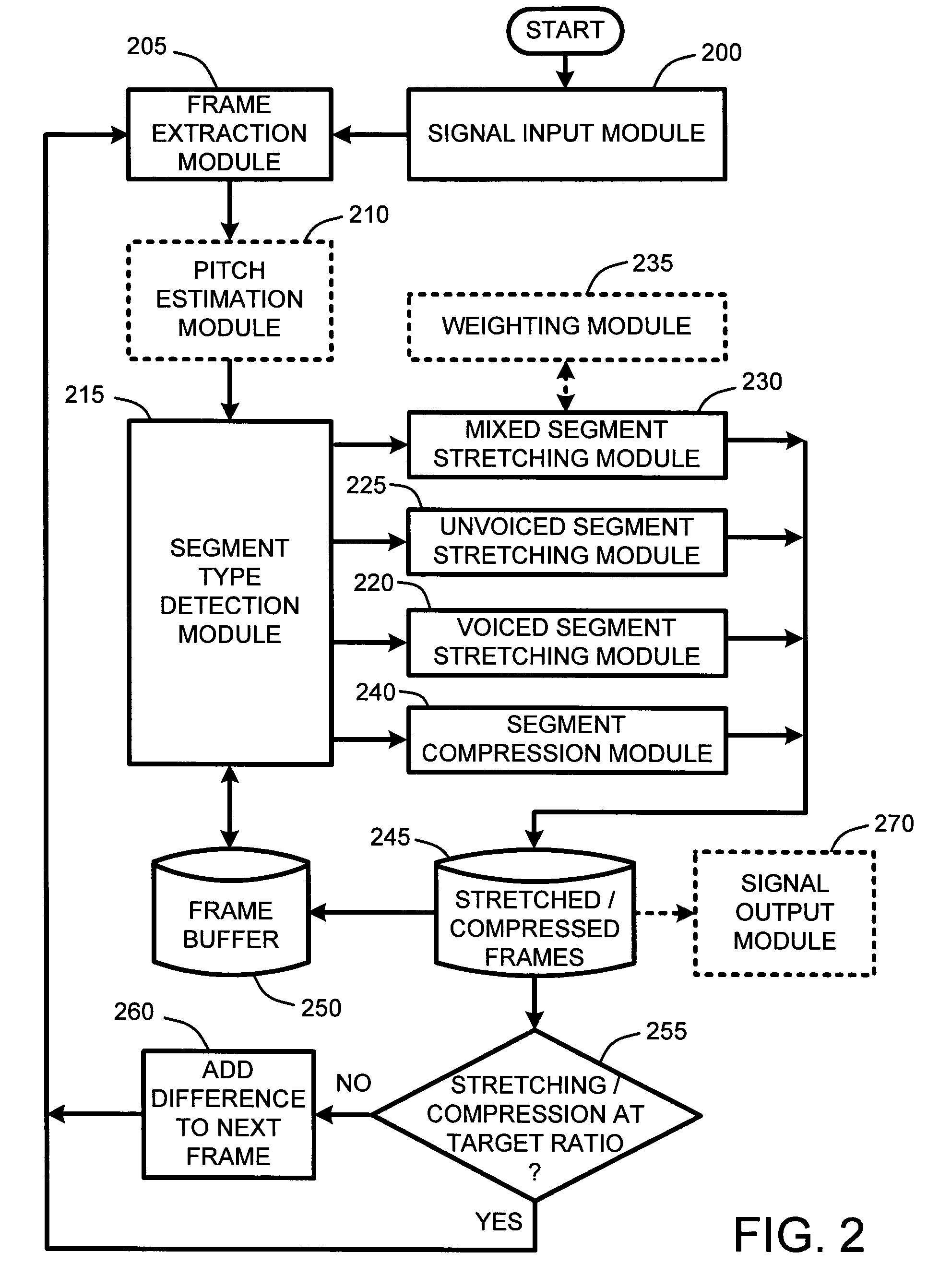
InactiveUS7337108B2Quality improvementImprove intelligibilitySpeech analysisCode conversionCompression methodSelf adaptive
An adaptive “temporal audio scaler” is provided for automatically stretching and compressing frames of audio signals received across a packet-based network. Prior to stretching or compressing segments of a current frame, the temporal audio scaler first computes a pitch period for each frame for sizing signal templates used for matching operations in stretching and compressing segments. Further, the temporal audio scaler also determines the type or types of segments comprising each frame. These segment types include “voiced” segments, “unvoiced” segments, and “mixed” segments which include both voiced and unvoiced portions. The stretching or compression methods applied to segments of each frame are then dependent upon the type of segments comprising each frame. Further, the amount of stretching and compression applied to particular segments is automatically variable for minimizing signal artifacts while still ensuring that an overall target stretching or compression ratio is maintained for each frame.
Owner:MICROSOFT TECH LICENSING LLC
Double compression unloadable screw system
InactiveUS20050143735A1Easy to compressIncrease heightInternal osteosythesisJoint implantsDouble compressionScrew system
A bone screw assembly for joining bone fragments having a screw shaft, a compression head and a sleeve. The compression head has external threads for providing double compression of the bone fragments. The sleeve extends along and around the screw shaft to provide reinforcing strength and alignment to the screw.
Owner:MILLENNIUM MEDICAL TECH INC
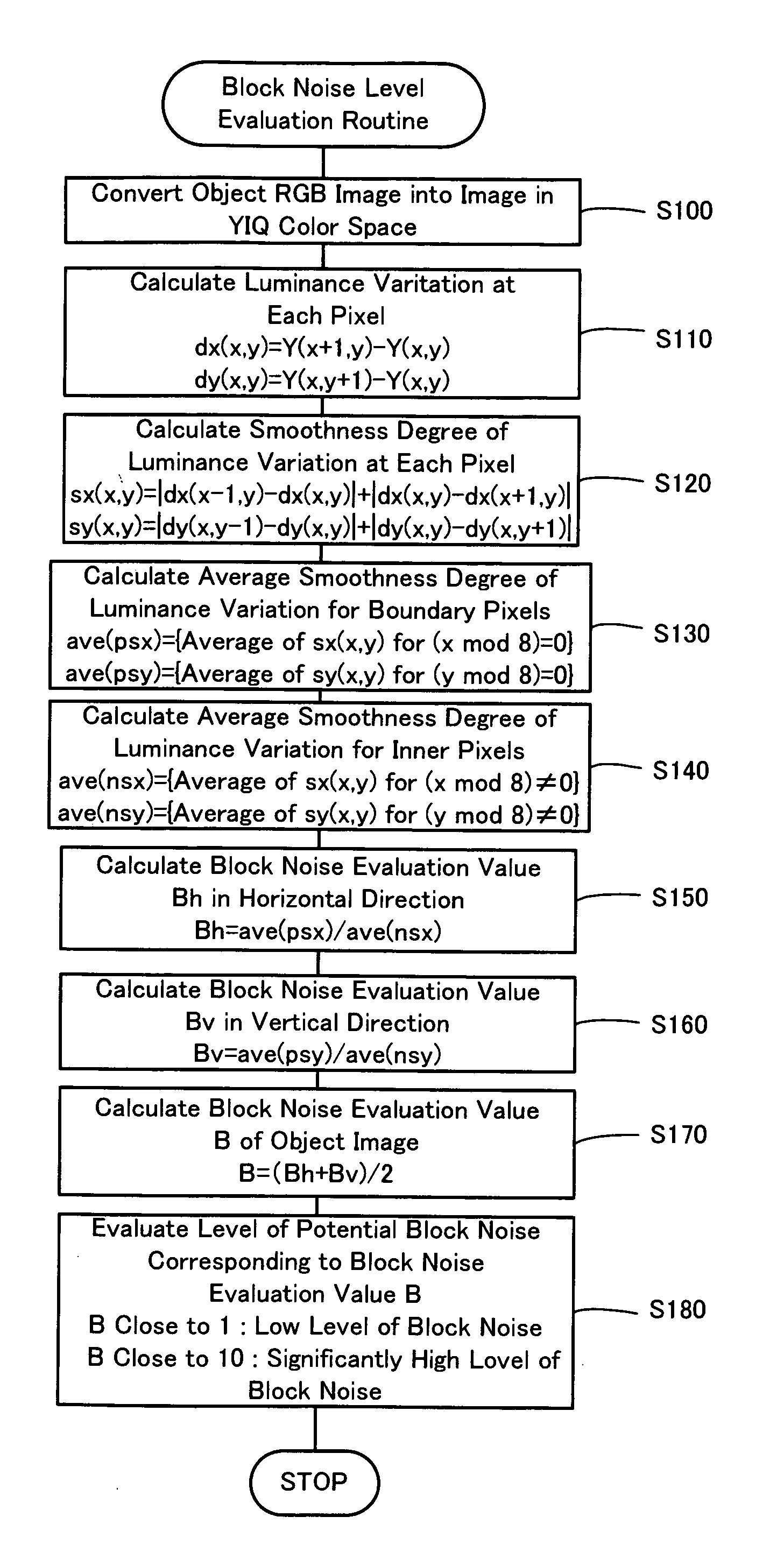
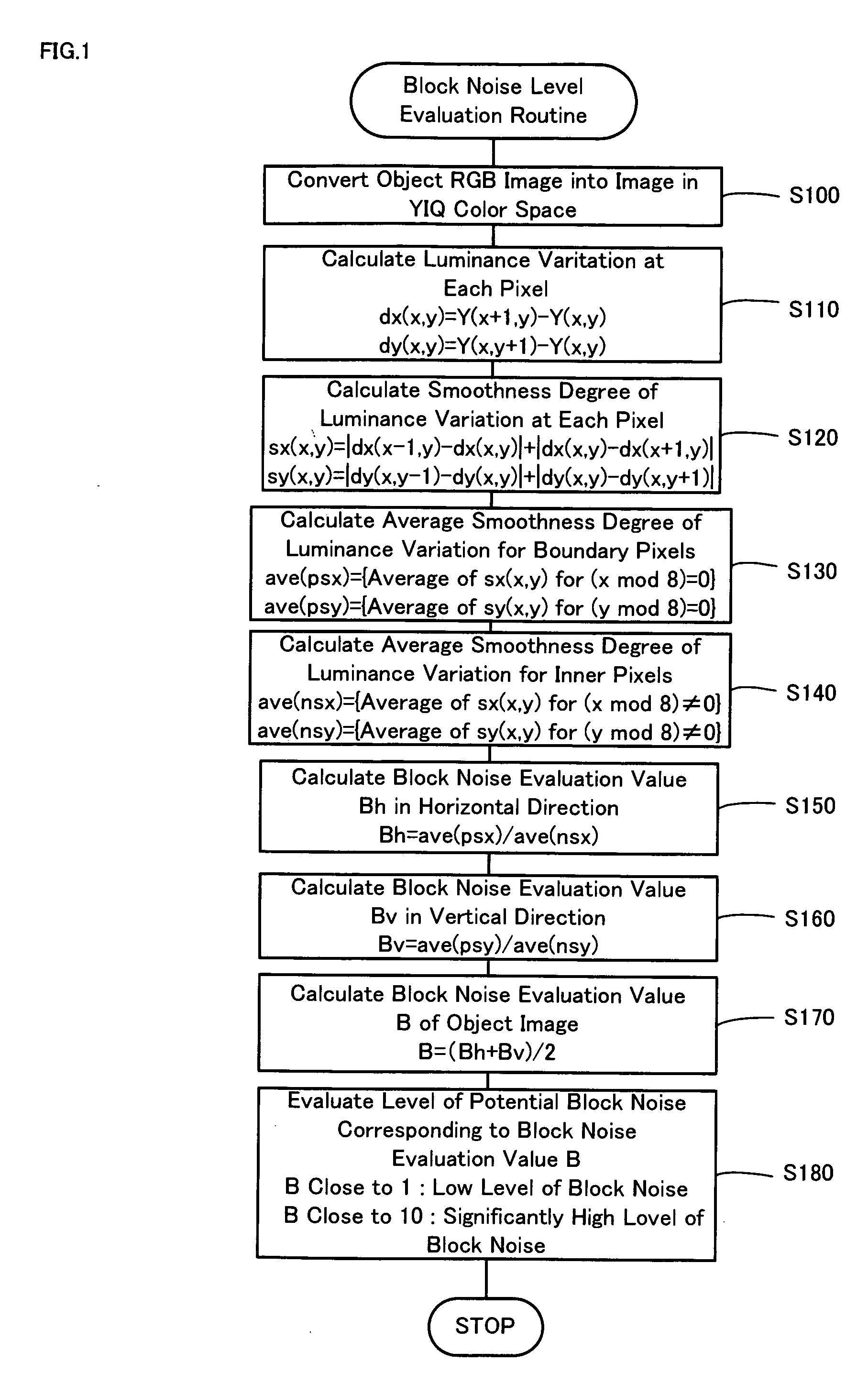
Block noise level evaluation method for compressed images and control method of imaging device utilizing the evaluation method
InactiveUS20060034531A1Reducing influence level of noiseReduce in quantityImage enhancementImage analysisPattern recognitionNoise level
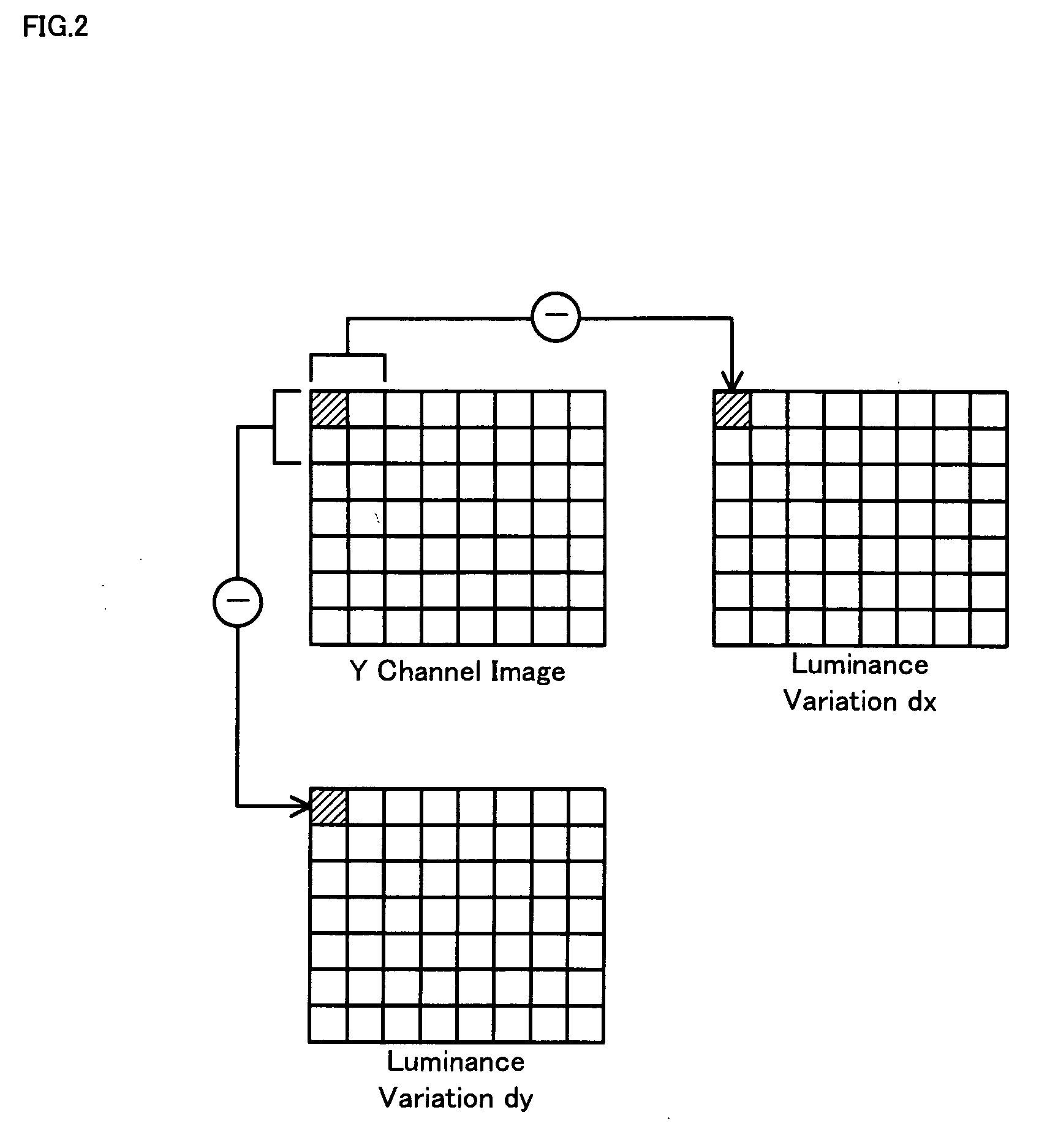
The technique of the invention converts an object RGB image into an image in a YIQ color space, calculates a luminance variation at each target pixel from Y channel values of the target pixel and an adjacent pixel adjoining to the target pixel, and computes a smoothness degree of luminance variation at the target pixel as summation of absolute values of differences between luminance variations at the target pixel and adjacent pixels. A block noise evaluation value B is obtained as a ratio of an average smoothness degree ave(psx), ave(psy) of luminance variation for boundary pixels located on each block boundary to an average smoothness degree ave(nsx), ave(nsy) of luminance variation for inner pixels not located on the block boundary. The block noise evaluation value B closer to 1 gives an evaluation result of a lower level of block noise, whereas the block noise evaluation value B closer to 10 gives an evaluation result of a higher level of block noise.
Owner:SEIKO EPSON CORP
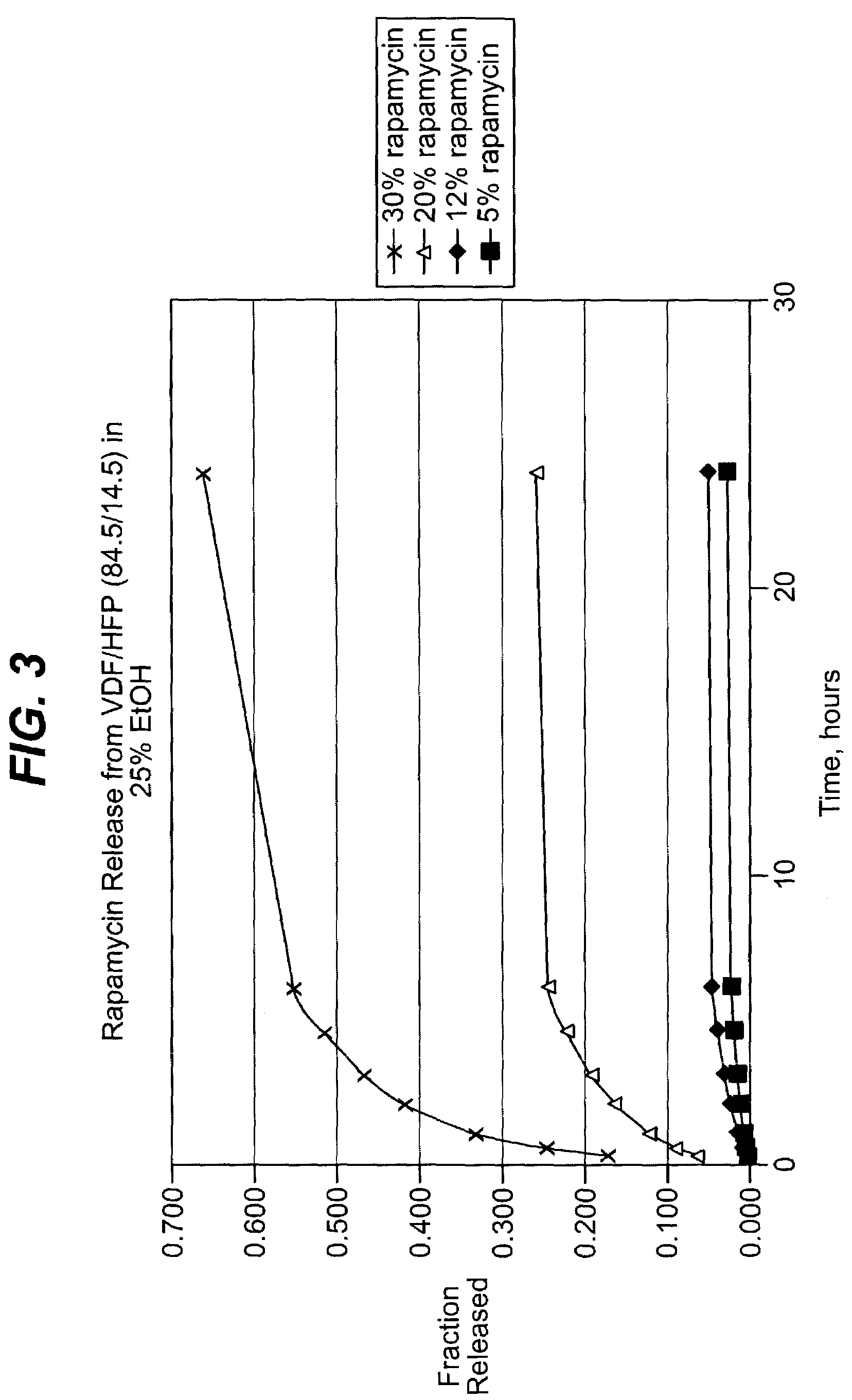
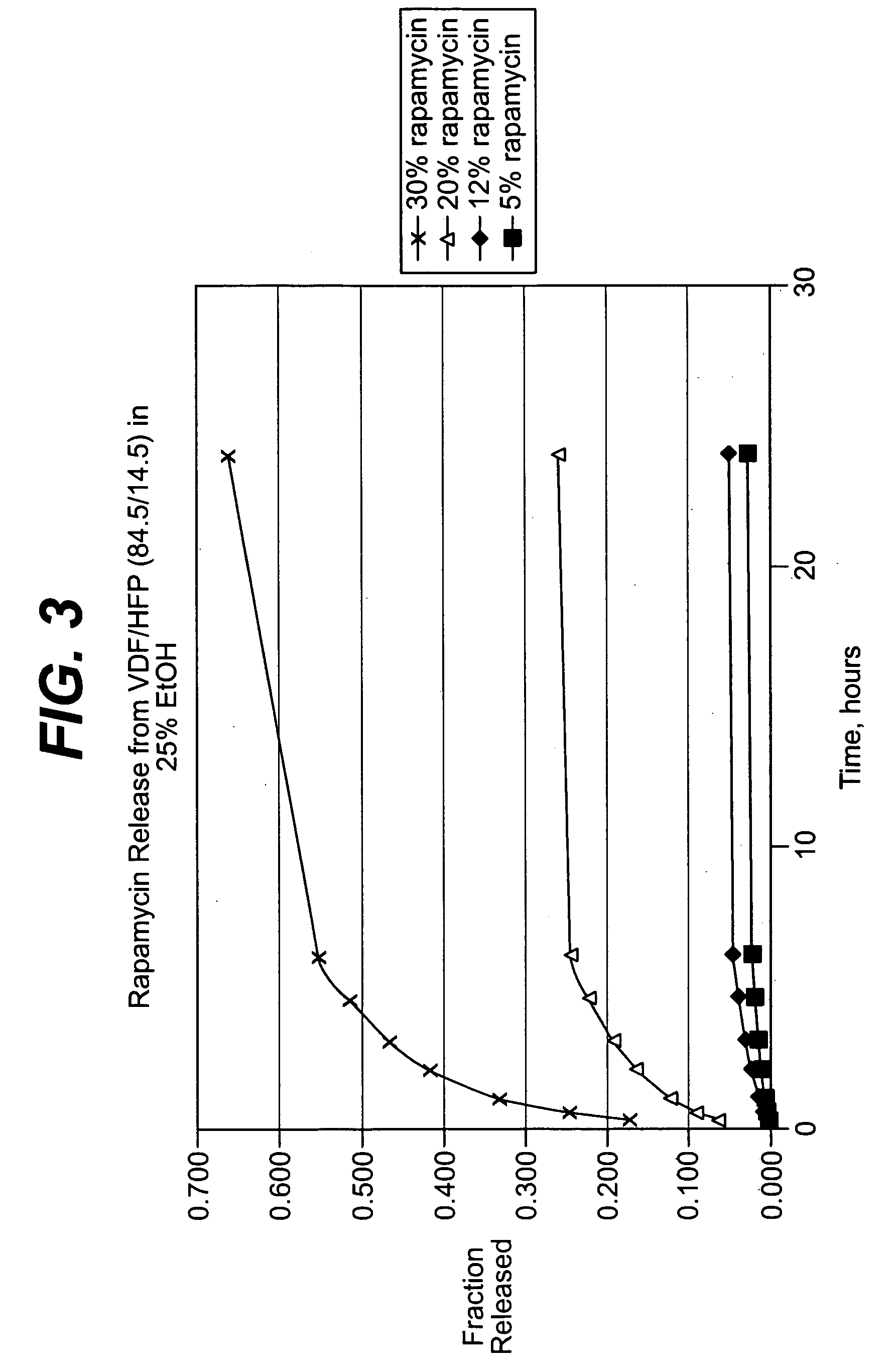
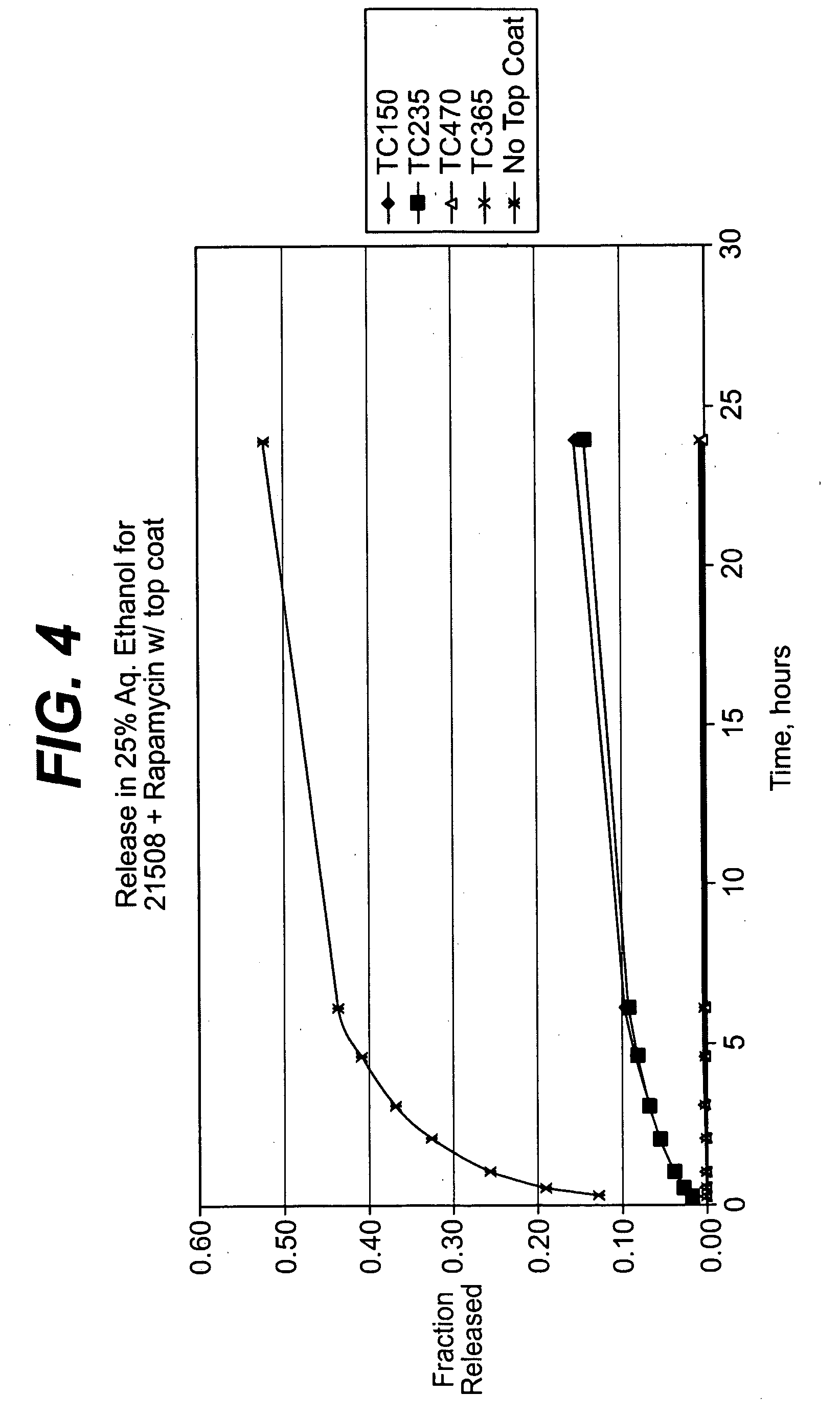
Solution formulations of sirolimus and its analogs for CAD treatment
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
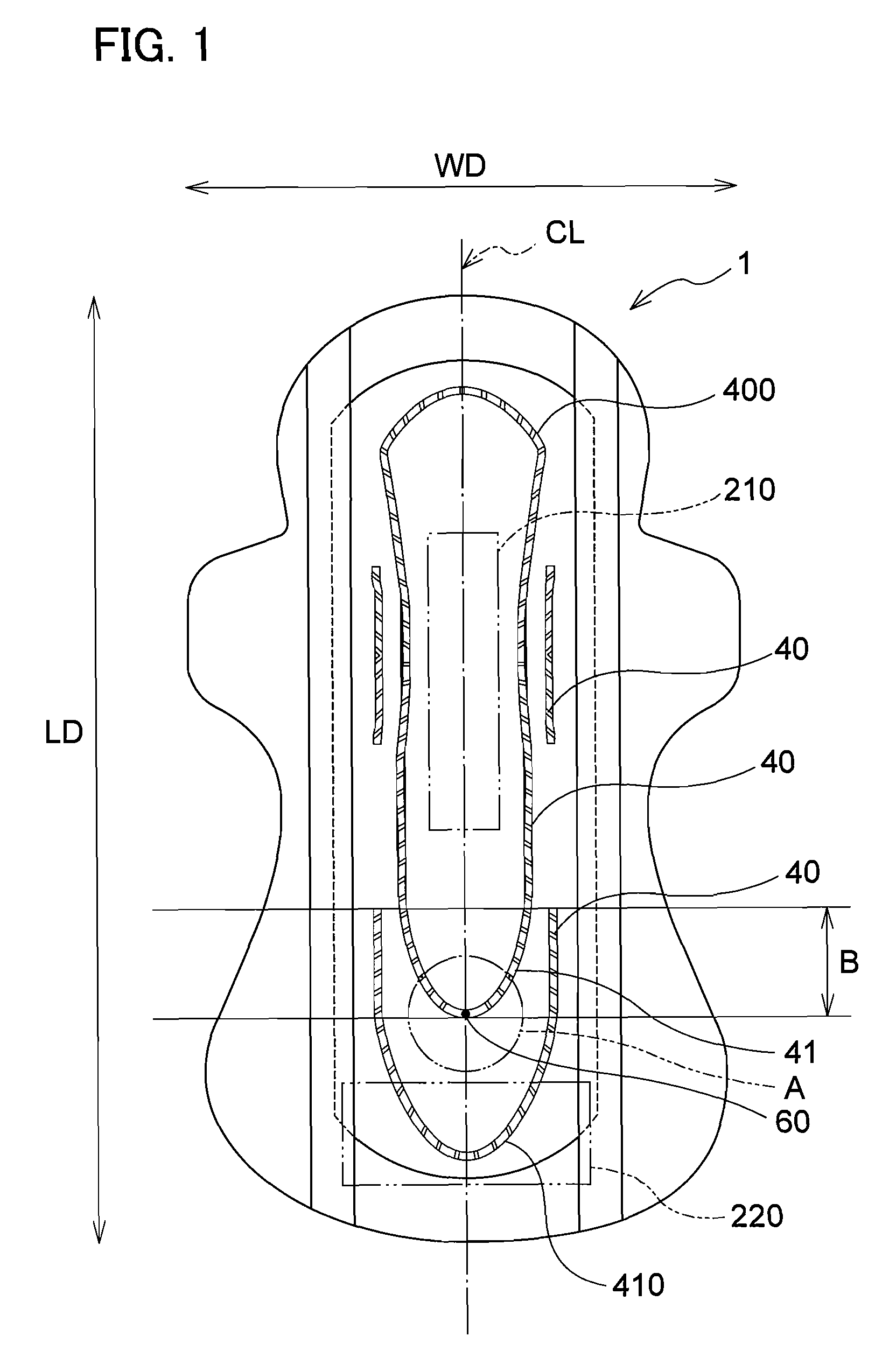
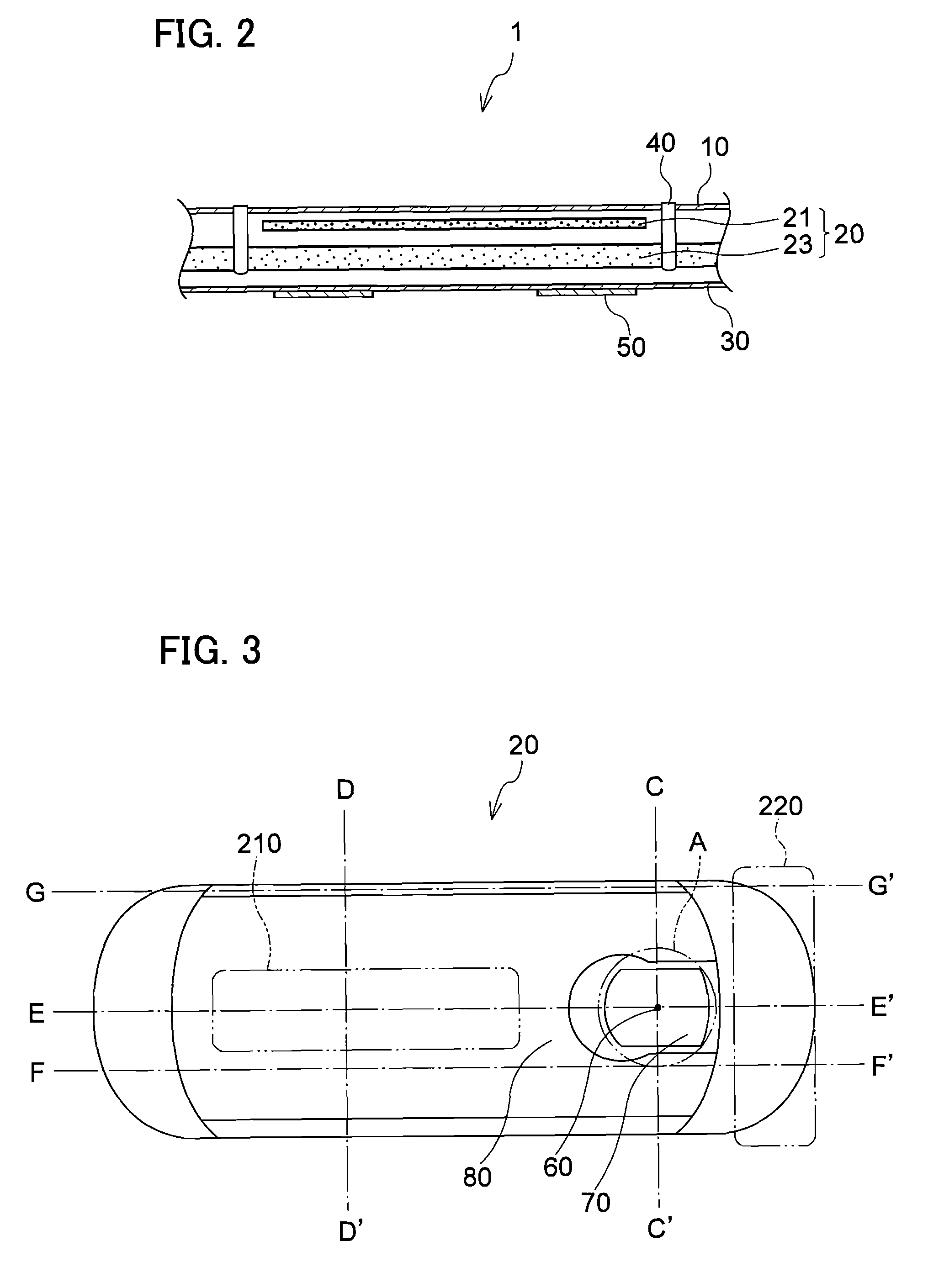
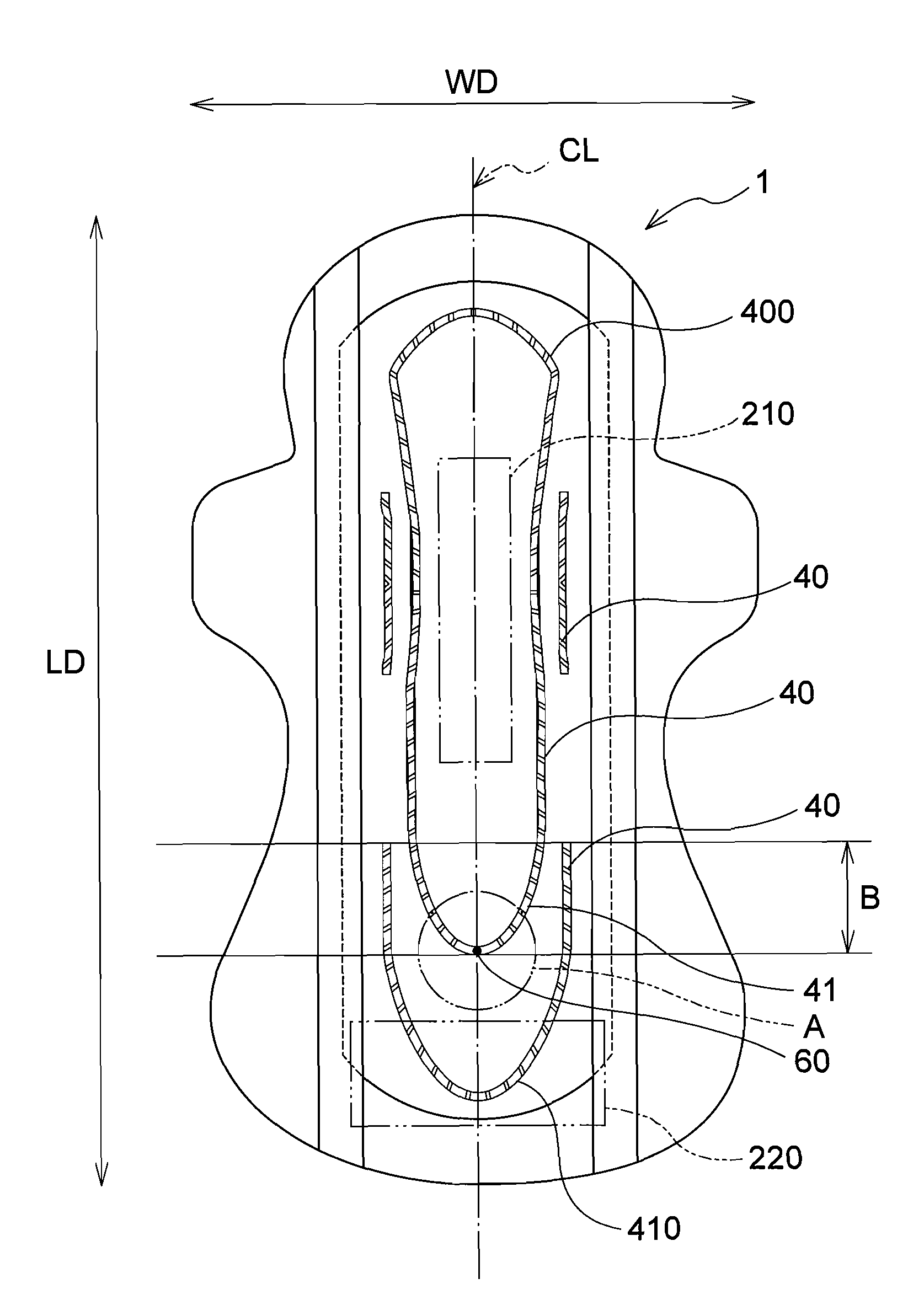
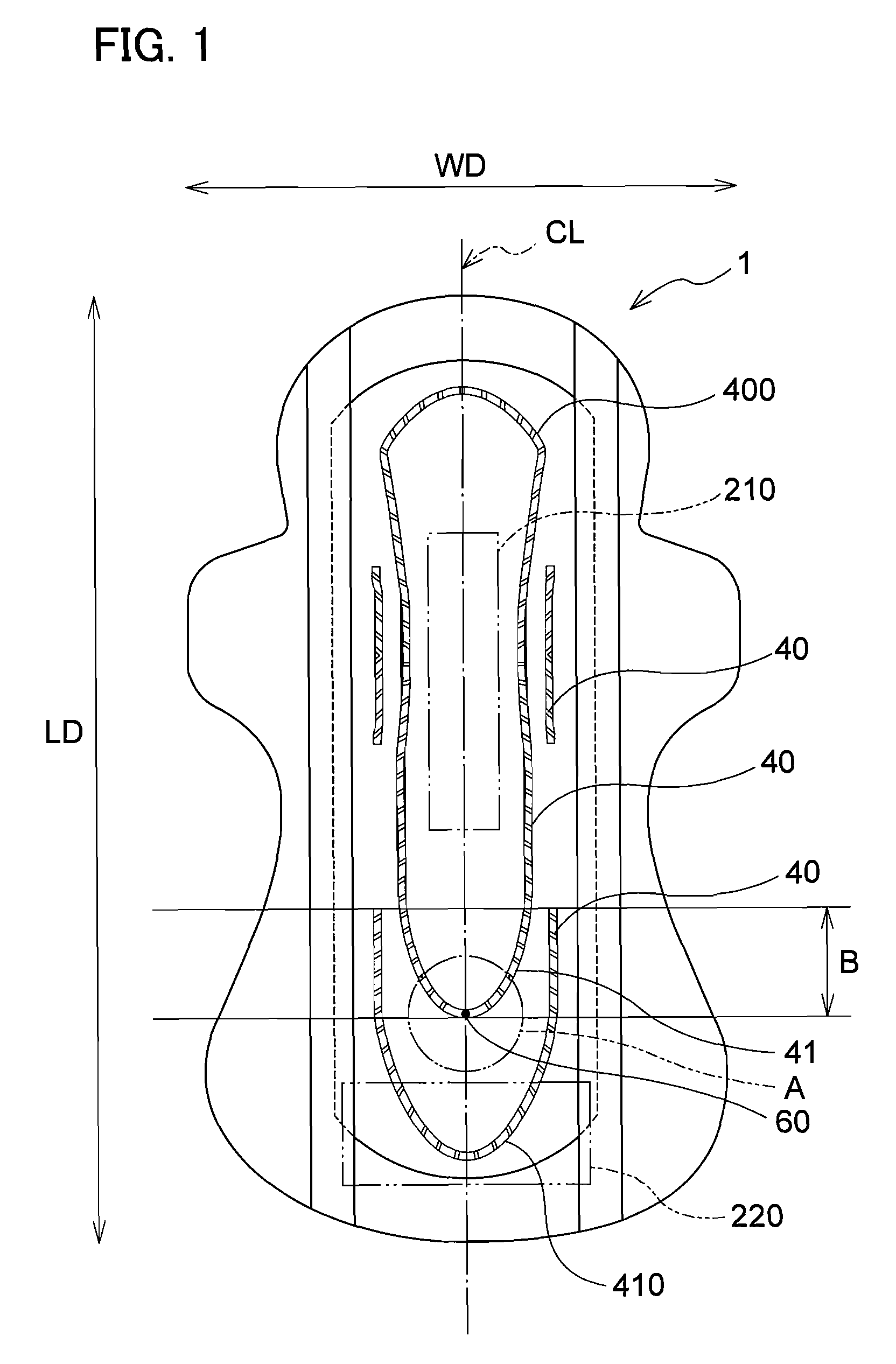
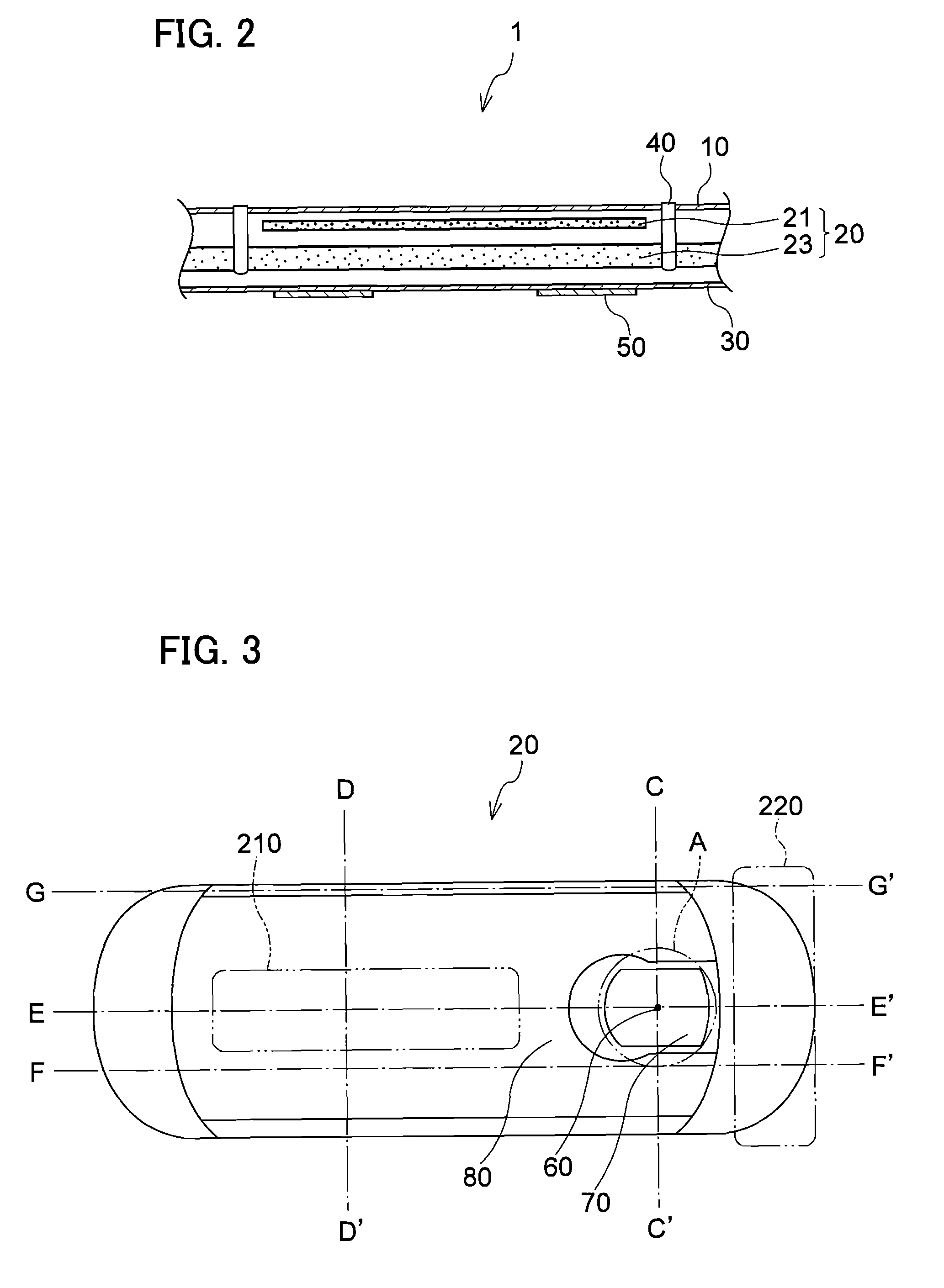
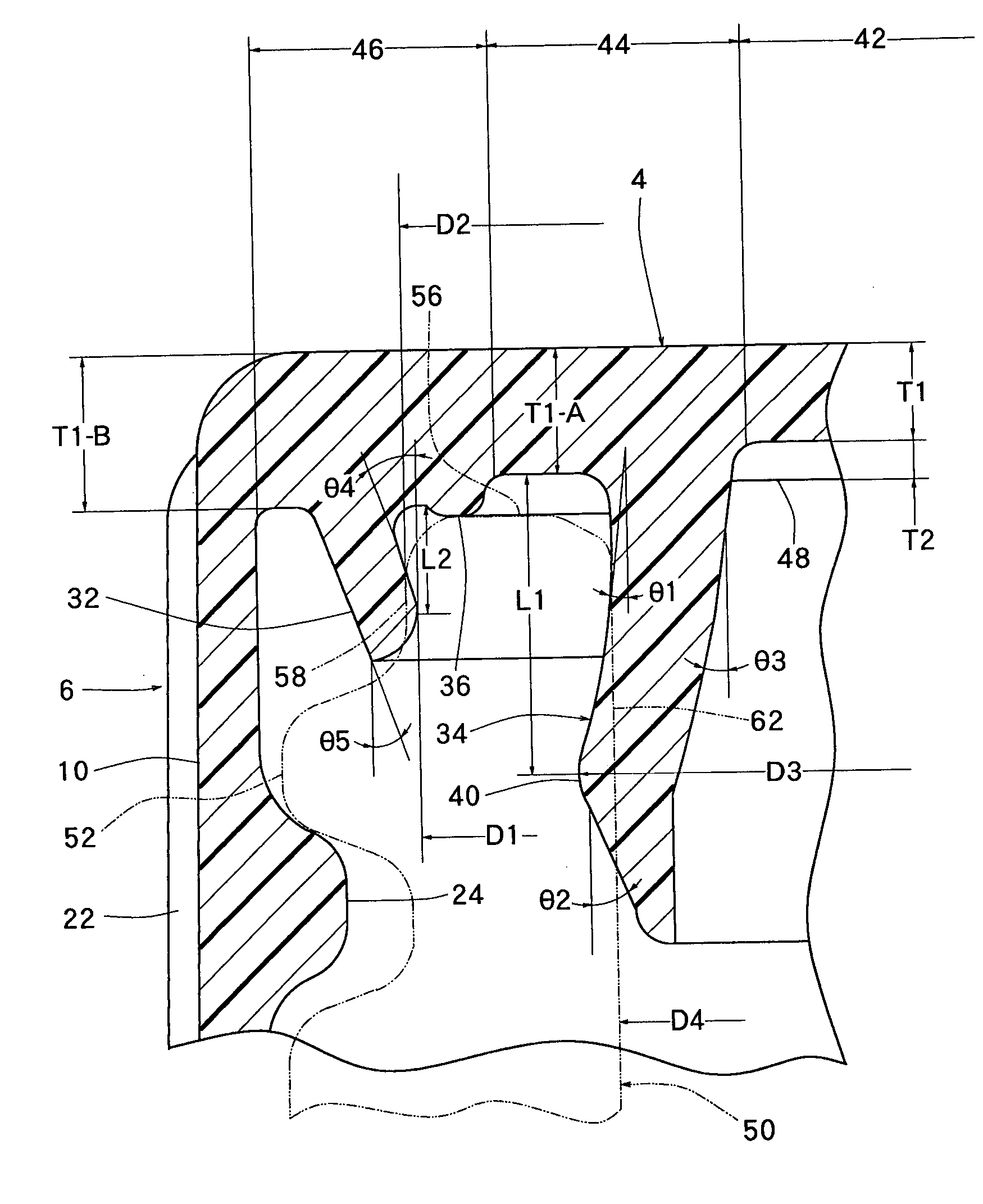
Absorbent article
InactiveUS20070073253A1Low elastic modulusAvoid separationSanitary towelsBaby linensEngineeringMechanical engineering
Owner:UNI CHARM CORP
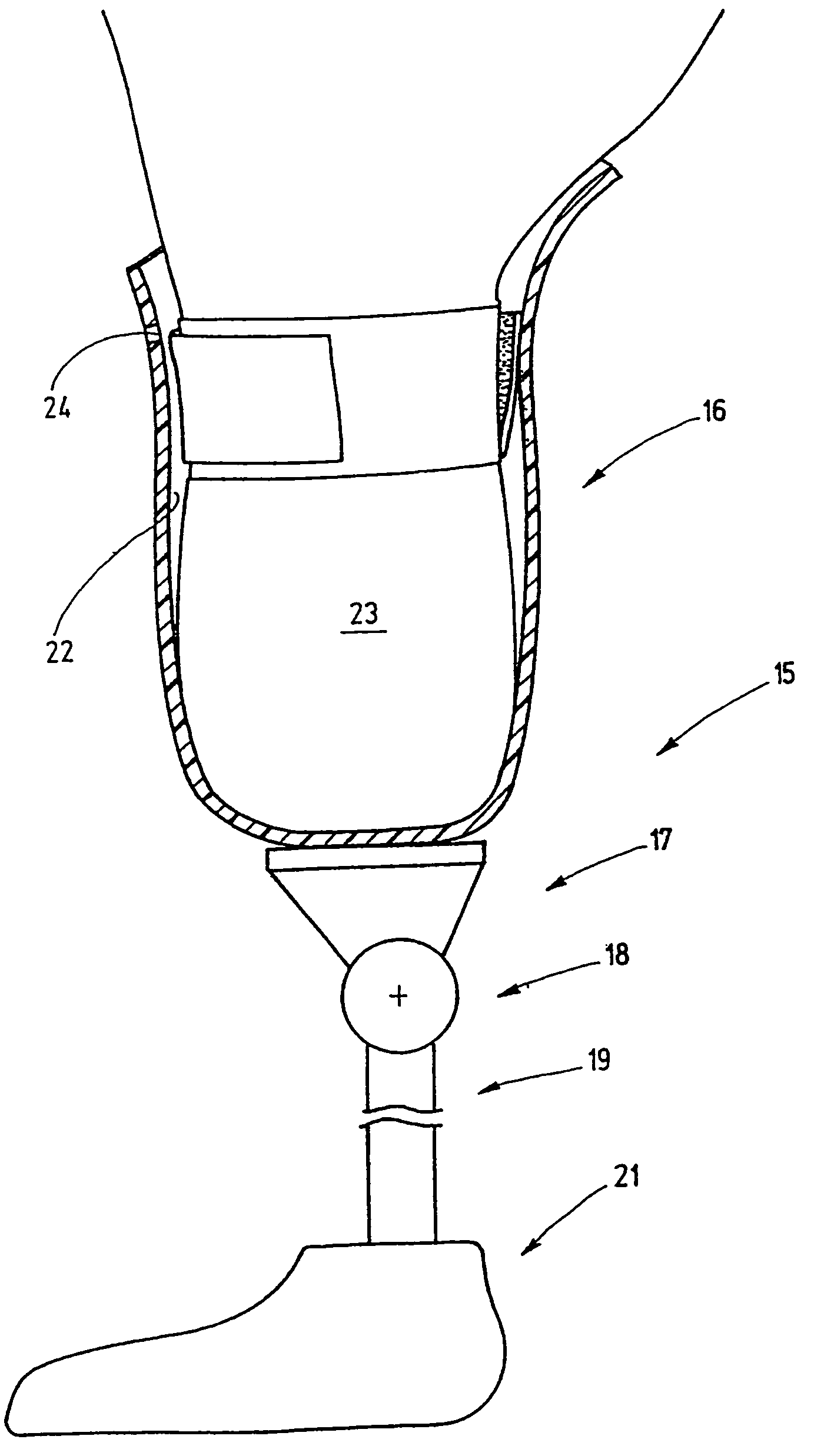
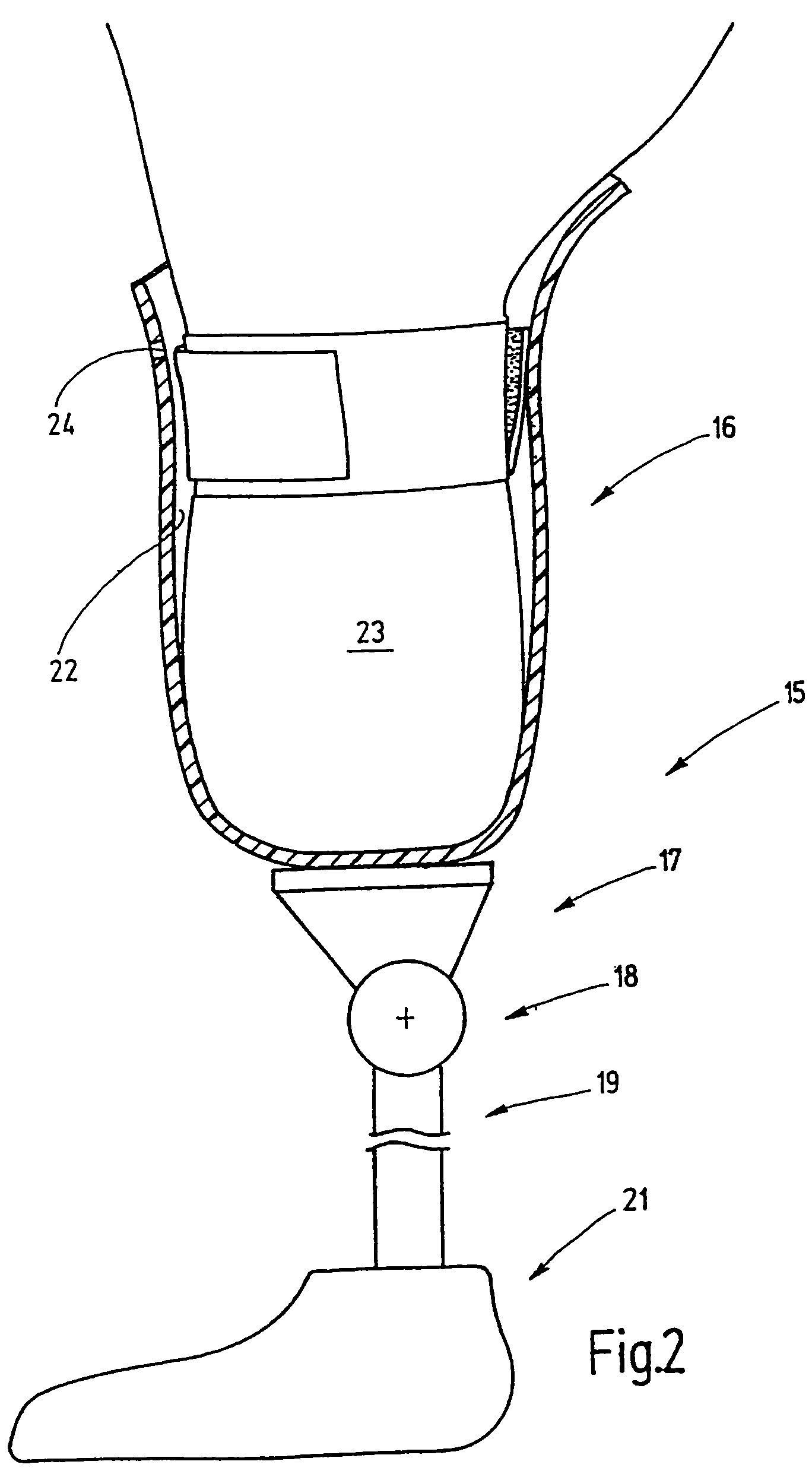
Seal arrangement for residual limb prosthetic socket
A prosthetic socket seal includes at least one radially projecting sealing lip which jointly with a base subtends an annular gap. This annular gap is vented on a proximal side by the external atmosphere, resulting in creation of a pressure gradient at the sealing lip that produces stronger compression of the sealing lip against the prosthetic socket with increasing partial vacuum on a distal side of the sealing lip.
Owner:KAUPTHING BANK
Spinal dynamic stabilization device
InactiveUS20090112266A1Reduce complexityShorten the timeSuture equipmentsInternal osteosythesisTranspedicular fixationBiomedical engineering
A spinal dynamic stabilization device for maintaining an anatomical height between two adjacent vertebras is provided. Each vertebra includes a spinous process and two symmetric pedicles. The spinal dynamic stabilization device includes a supporting member, at least one anchoring member, and at least one connecting member. The supporting member is disposed between the spinous processes. The anchoring member is fixed in one of the vertebra via one of the pedicles. The connecting member connects the supporting member to the anchoring member, fixing a relative position between the supporting member and the anchoring member, further fixing a relative position between the vertebras.
Owner:IND TECH RES INST
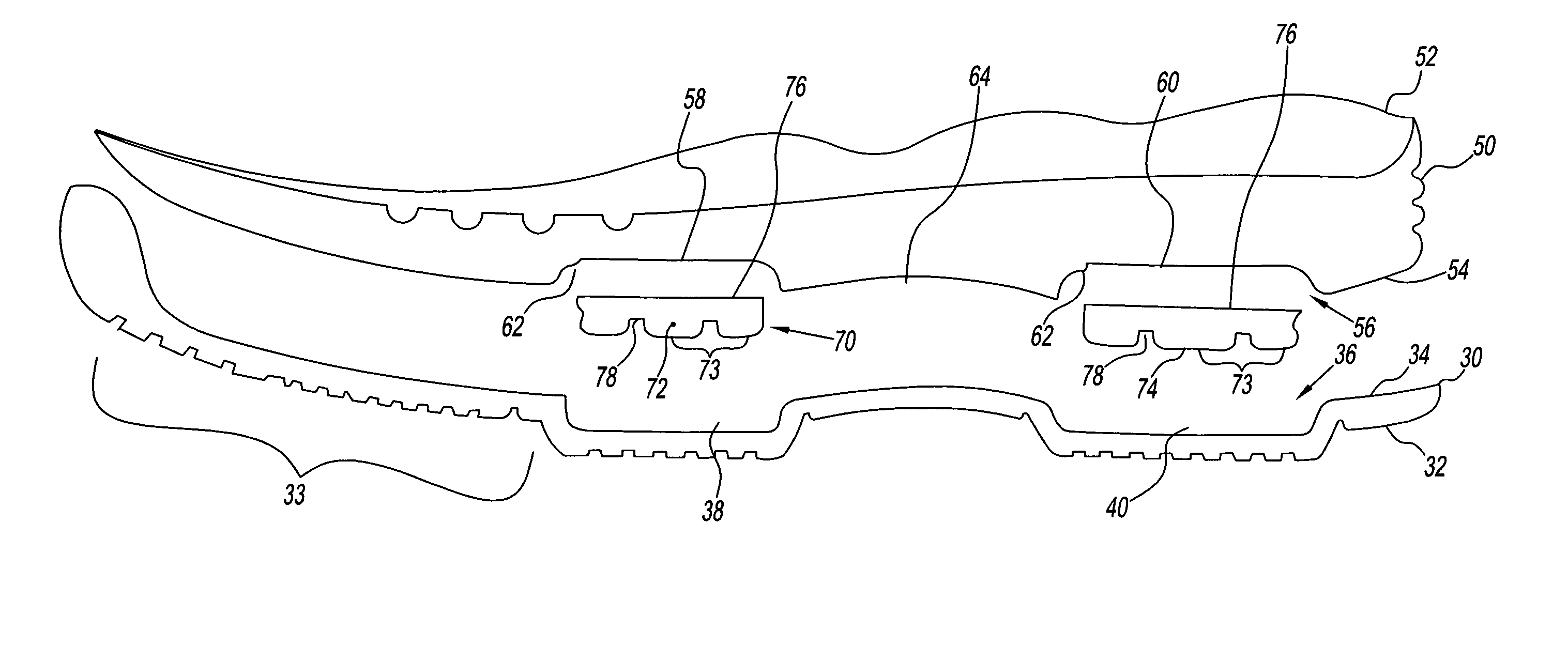
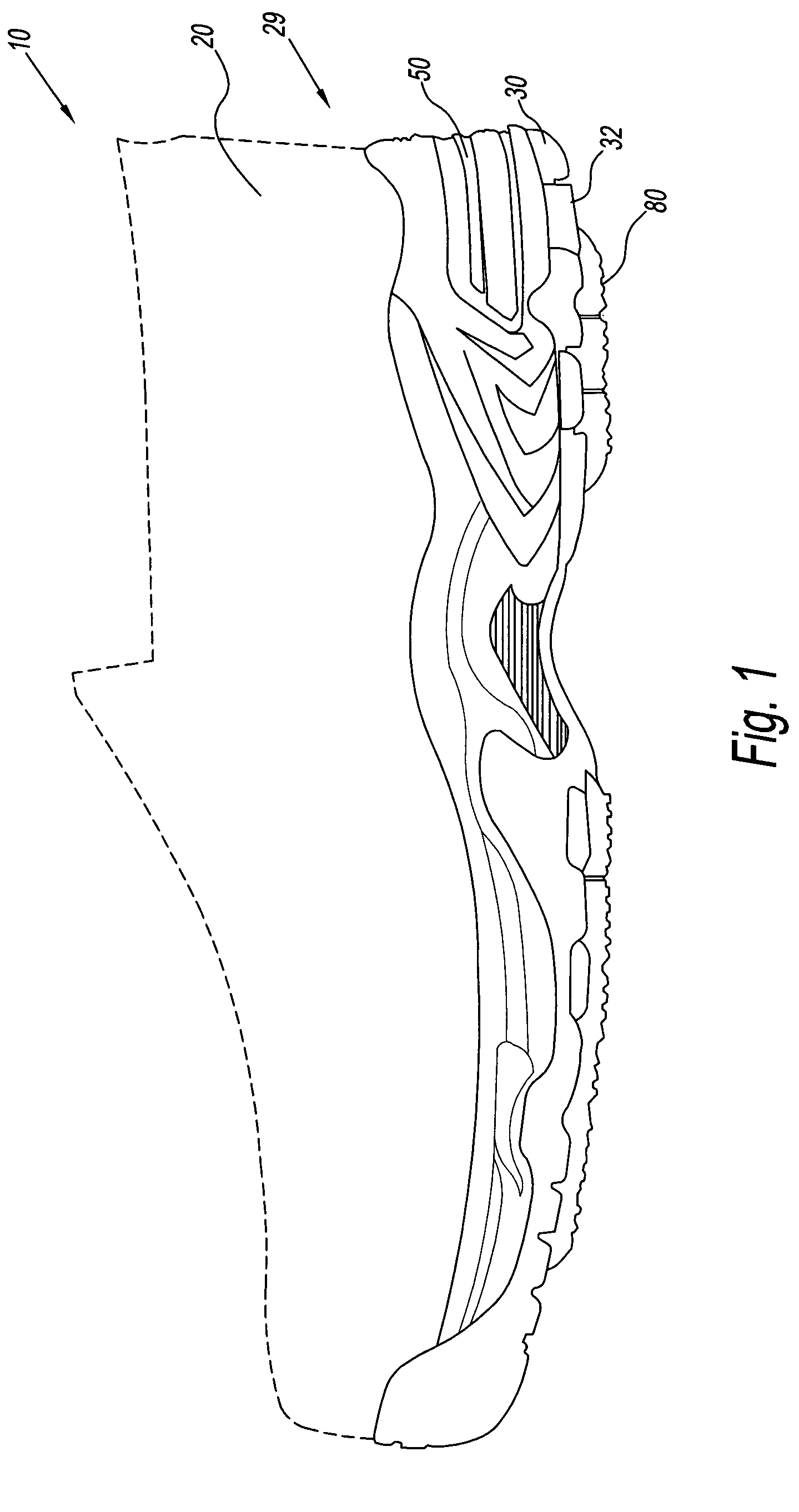
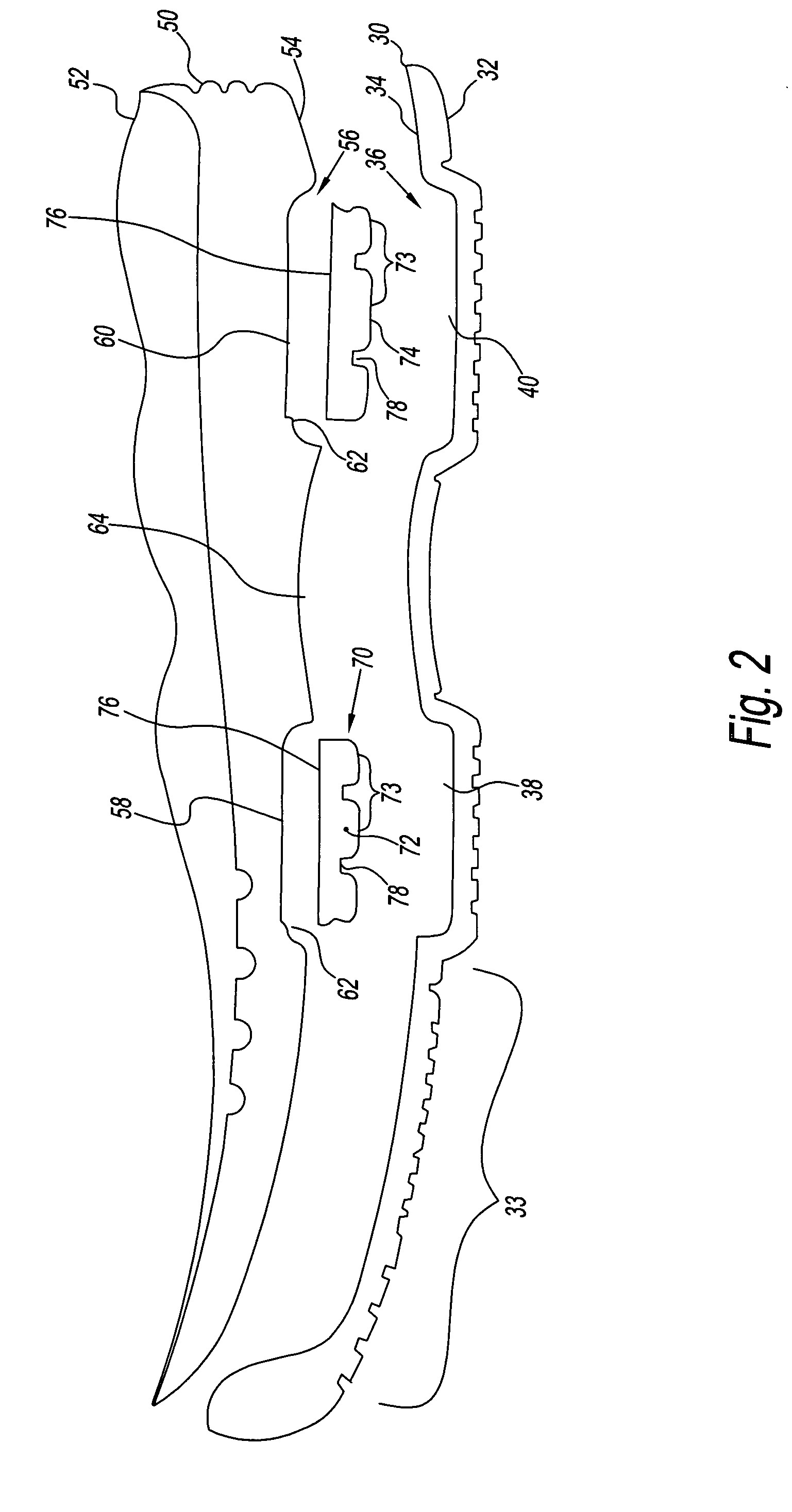
Footwear system
Owner:TRANSFORM SR BRANDS LLC
Interbody fusion system with intervertebral implant retention assembly
ActiveUS8187329B2Effective protectionLower profile of implantSpinal implantsFastenersPosterior instrumentationEngineering
The present disclosure is directed towards a biomechanical implant and anterior, lateral or posterior instrumentation construct. The construct may be of unitary or modular construction, whereby a single molded construction can form the entire assembly, in which case the through holes may be adapted to receive a metallic insert for screw fixation; or alternatively be of a modular construction wherein the anterior / lateral instrumentation and intervertebral spacer are designed for removable locking engagement, one with the other, for insertion by the surgeon as a unitary construct. A unique feature of the construct resides within the instrumentation construction, whereby a single opening formed therein permits two bone screws, or the like fastener device, to be positioned within both the superior and inferior vertebral bodies surrounding the spacer implant, or, for example in the case of a corpectomy or diskectomy with cage insertion, wherein two screws can be fixed within a single vertebral body through a single through hole, and wherein the bone screws are constructed and arranged to cooperate with the retention plate so as to provide locking engagement, one to the other, with the retention plate, upon final fixation thereof. Screw retention elements of alternative shape, based upon the choice of vertical or horizontal orientation, based upon an opened figure eight design, are provided for insertion in a groove formed in the borehole of the instrumentation plate which allows insertion of each fixation element but will prevent a loosened fixation element from falling out of the plate.
Owner:SPARTAN CAGE HLDG
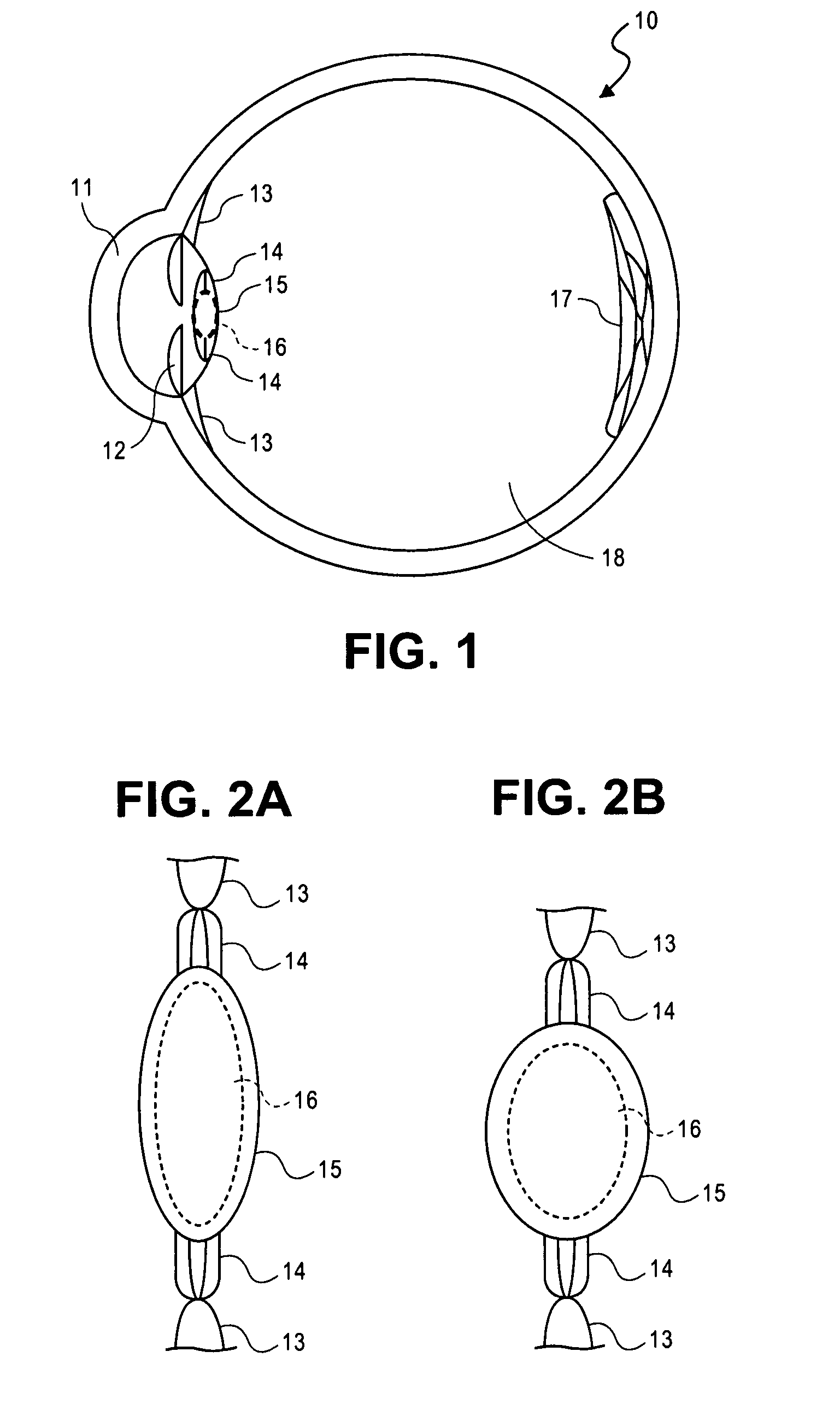
Accommodating intraocular lens system having circumferential haptic support and method
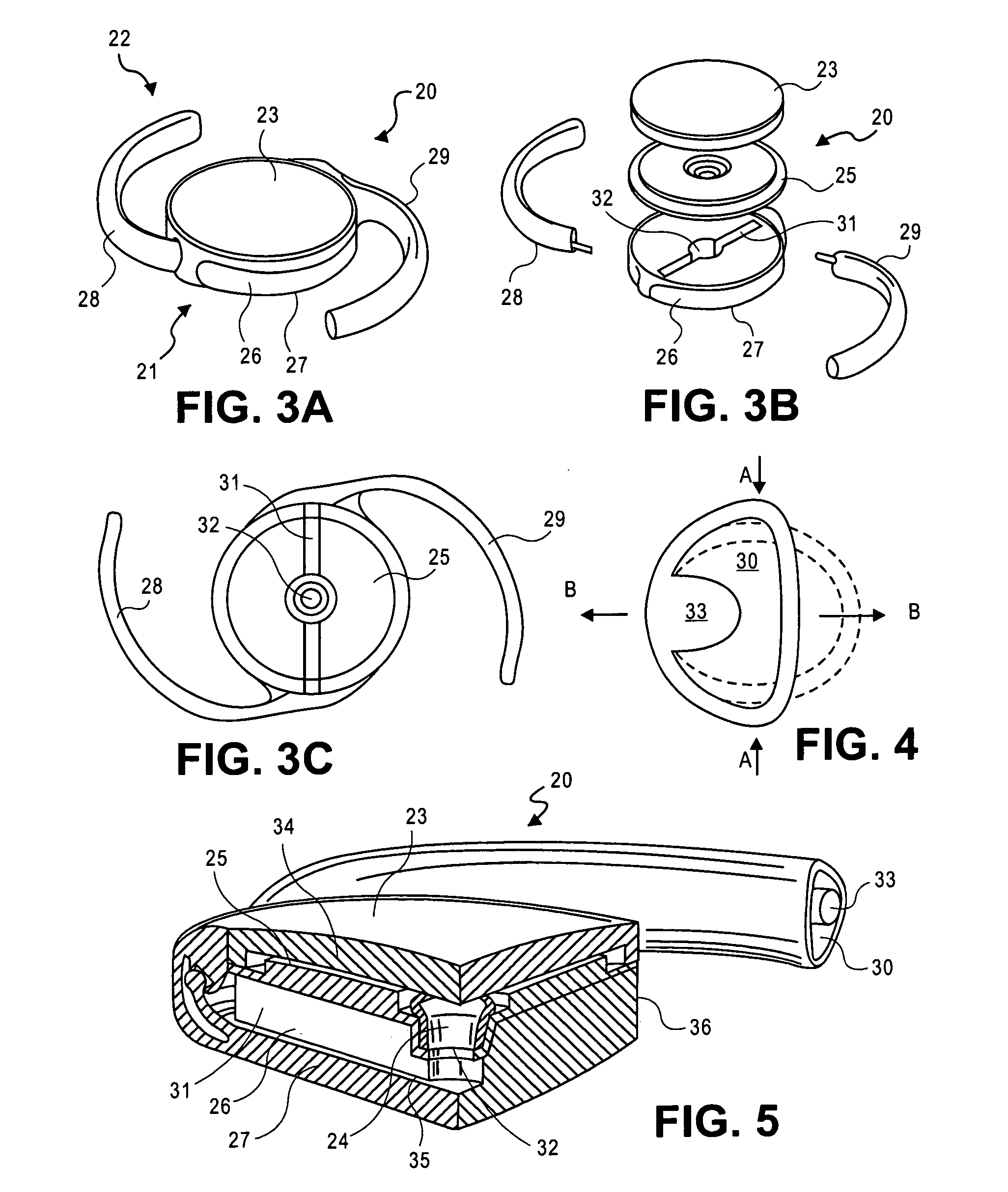
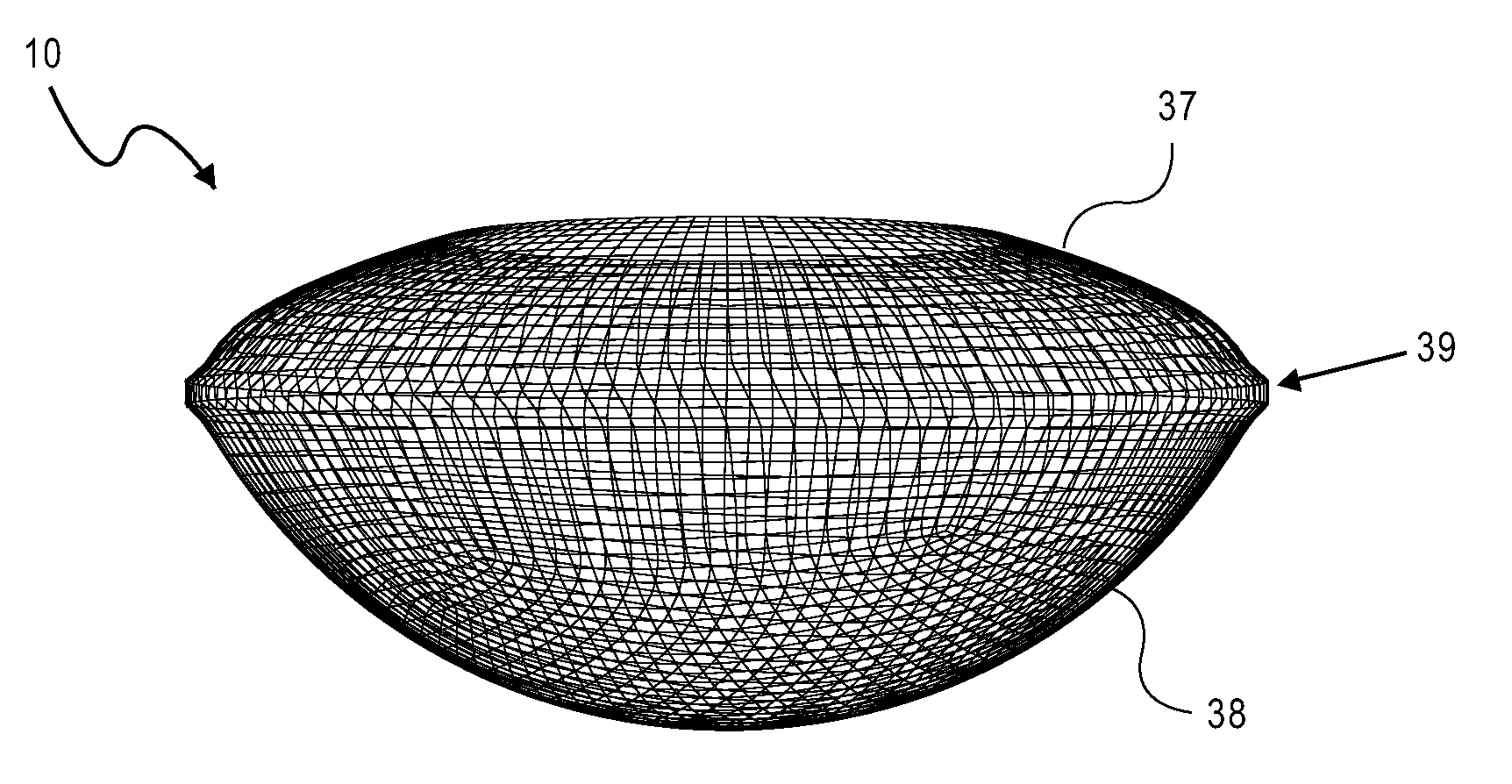
ActiveUS20070203578A1Reduce compressionIncrease the internal volumeIntraocular lensIntraocular lensOptical power
An accommodating intraocular lens includes an optic portion a haptic portion and a backstop. The optic portion of the lens includes an actuator that deflects a lens element to alter the optical power of the lens responsive to forces applied to the haptic portion of the lens by contraction of the ciliary muscles. Forces applied to the haptic portion may result in fluid displacements from or to the haptic portion from the actuator. The backstop provides support to the haptic so that bulk translation of the haptic is prevented in response to the forces applied by the capsular sac.
Owner:ALCON INC
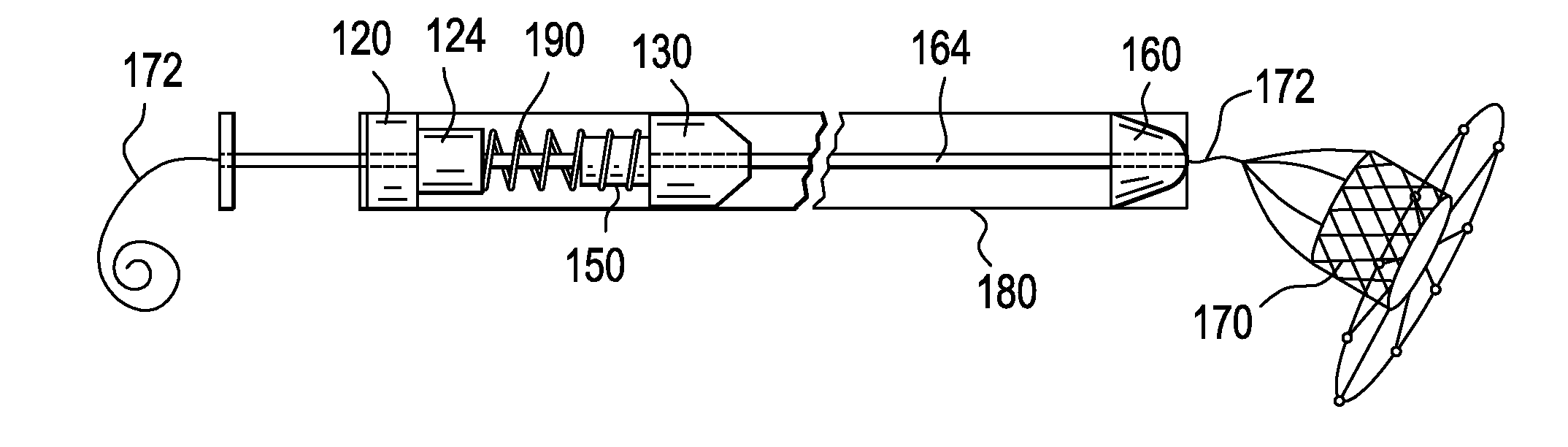
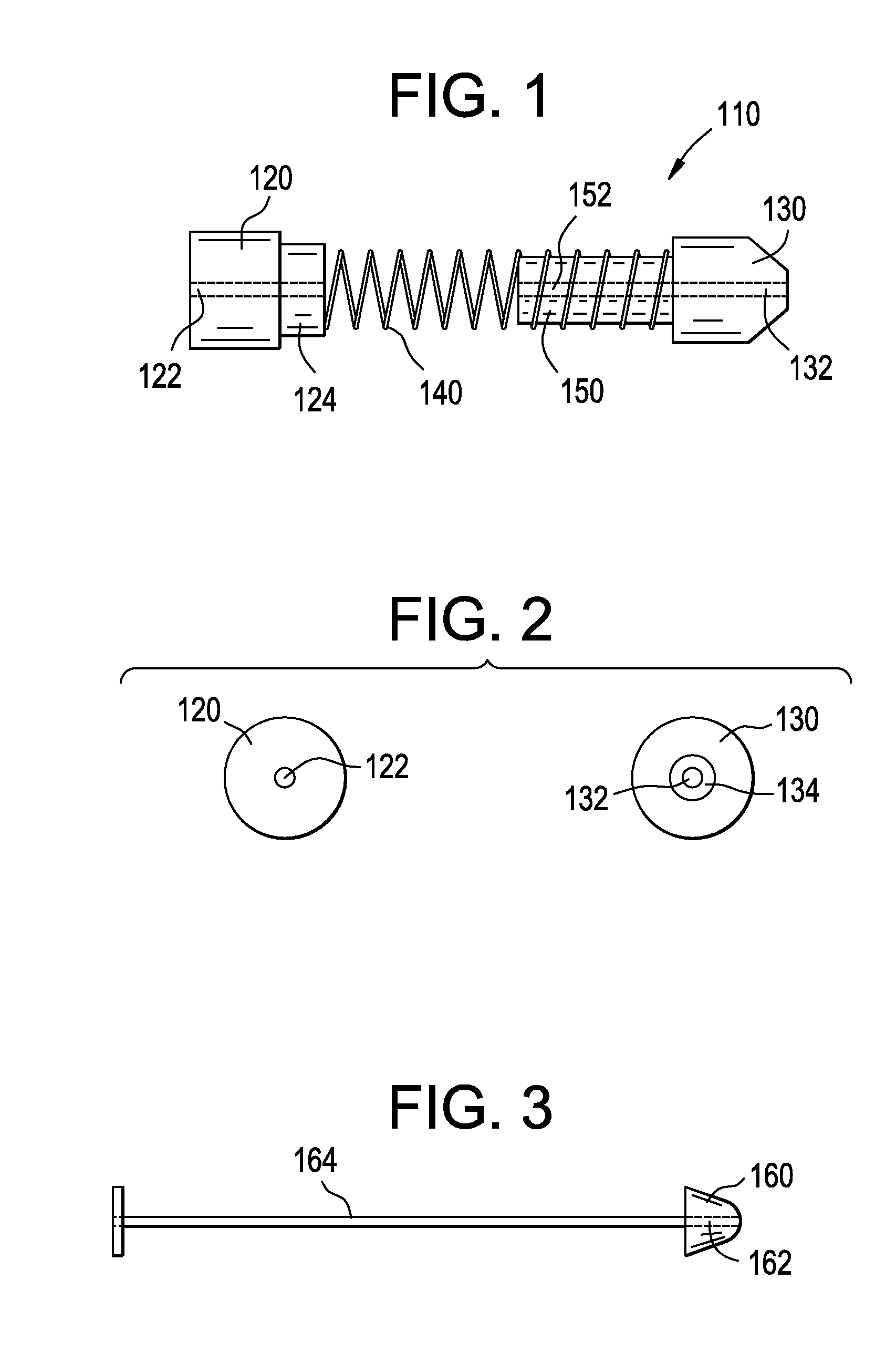
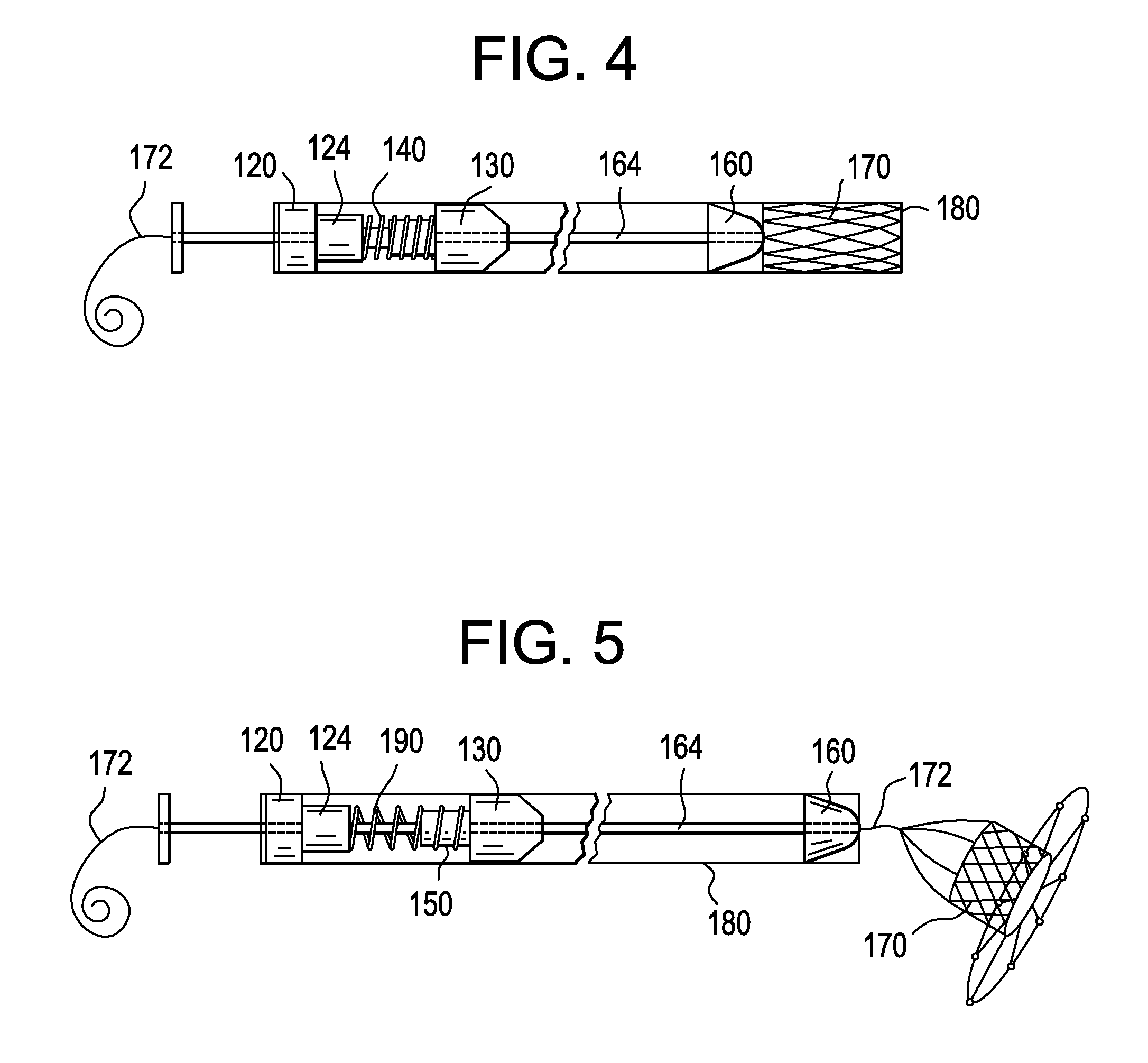
Deployment Compensator for Transcatheter Valve Delivery
Owner:TENDYNE HLDG
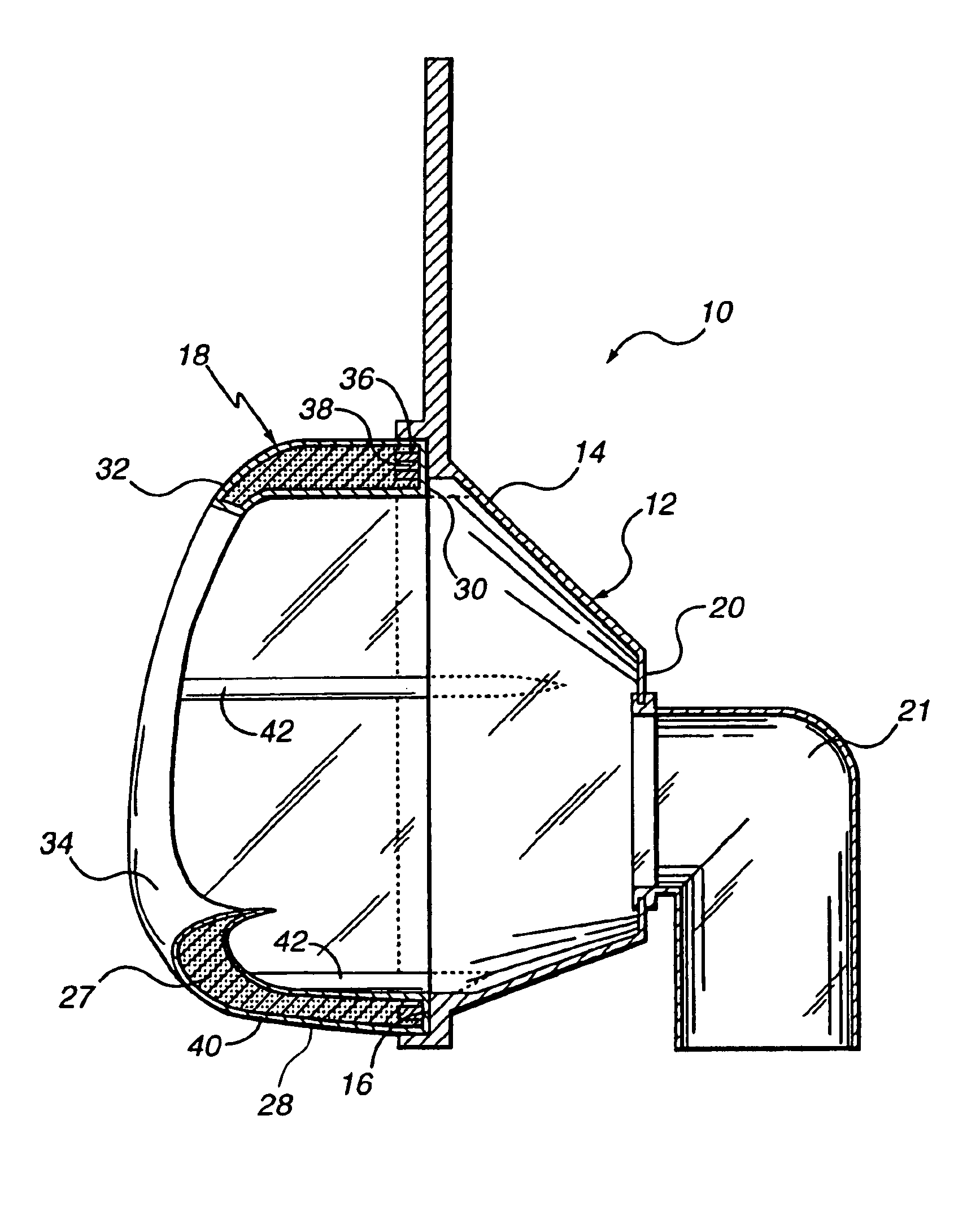
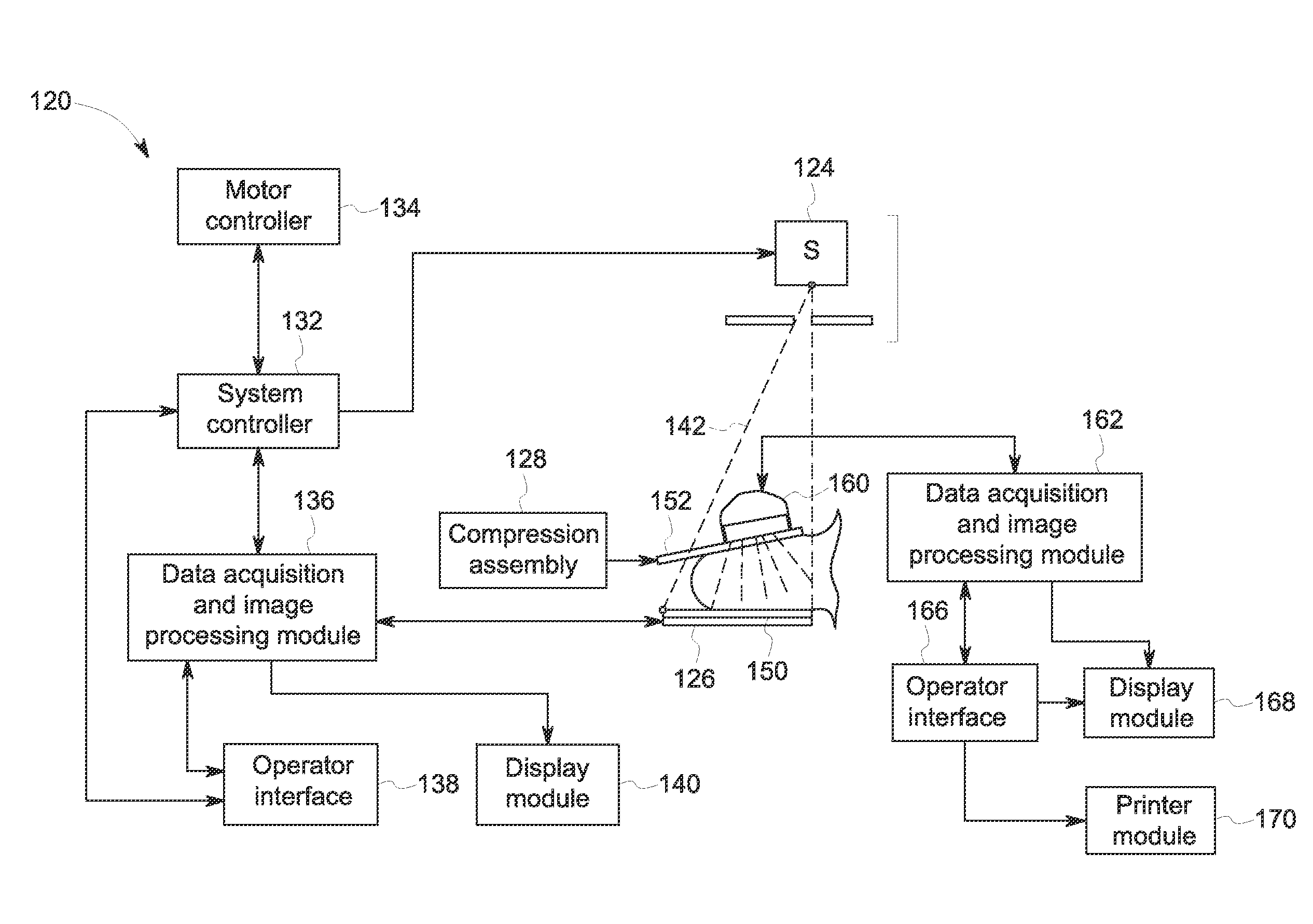
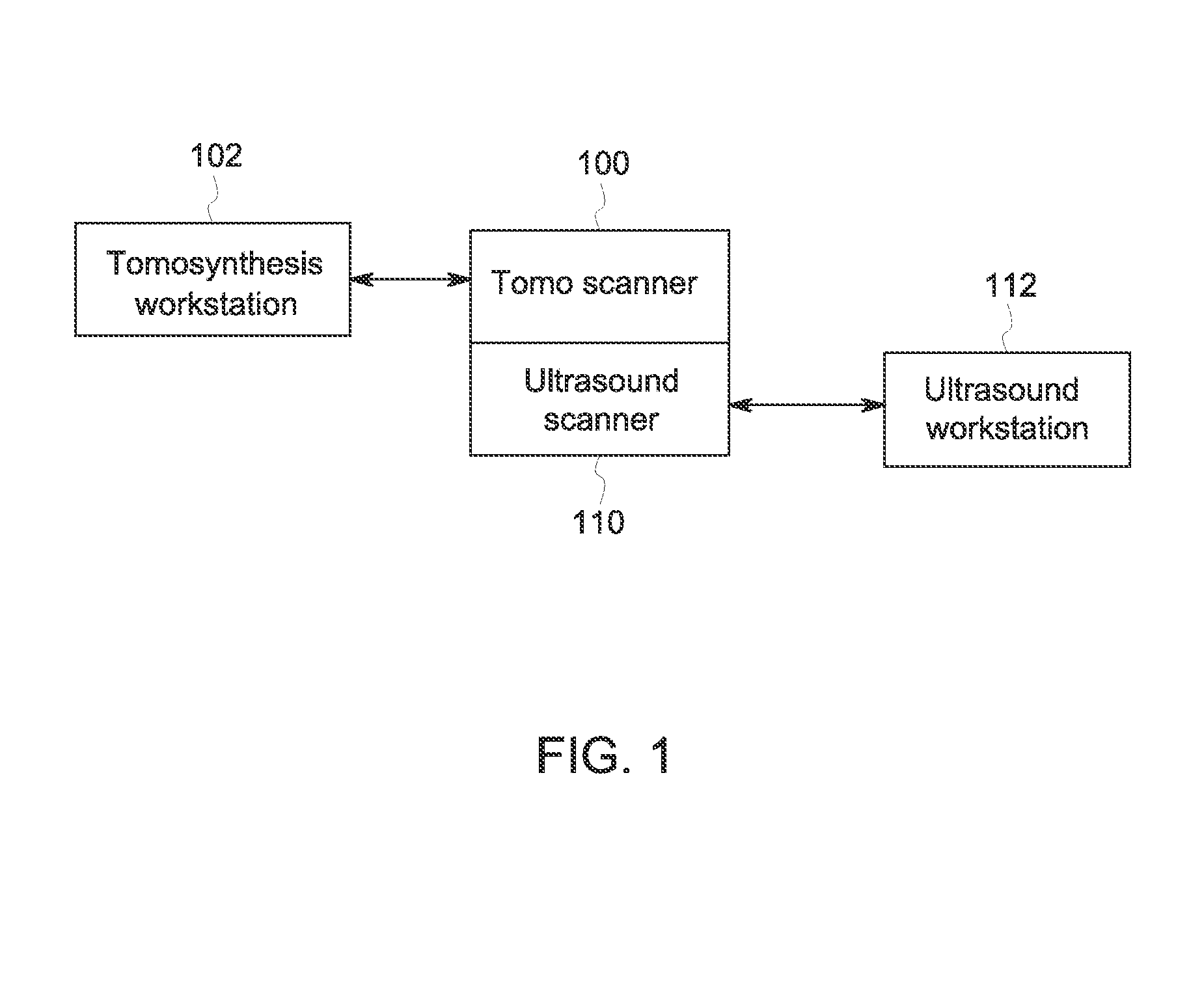
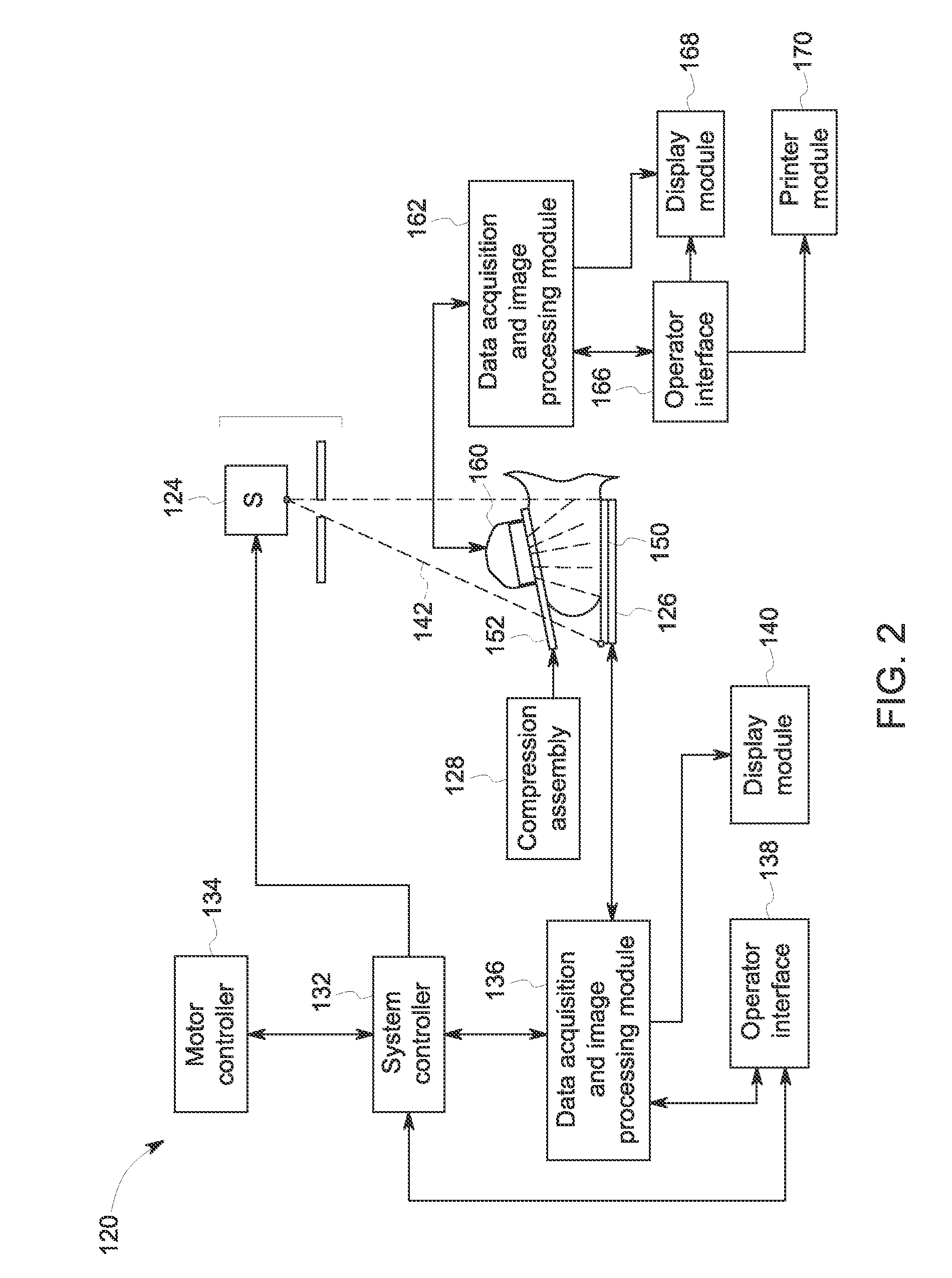
Breast imaging method and system
ActiveUS20160166234A1Reduce compressionOrgan movement/changes detectionTomosynthesisTomosynthesisUltrasound imaging
An ultrasound scan probe and support mechanism are provided for use in a multi-modality mammography imaging system, such as a combined tomosynthesis and ultrasound imaging system. In one embodiment, the ultrasound components may be positioned and configured so as not to interfere with the tomosynthesis imaging operation, such as to remain out of the X-ray beam path. Further, the ultrasound probe and associated components may be configured to as to move and scan the breast tissue under compression, such as under the compression provided by one or more paddles used in the tomosynthesis imaging operation.
Owner:GENERAL ELECTRIC CO
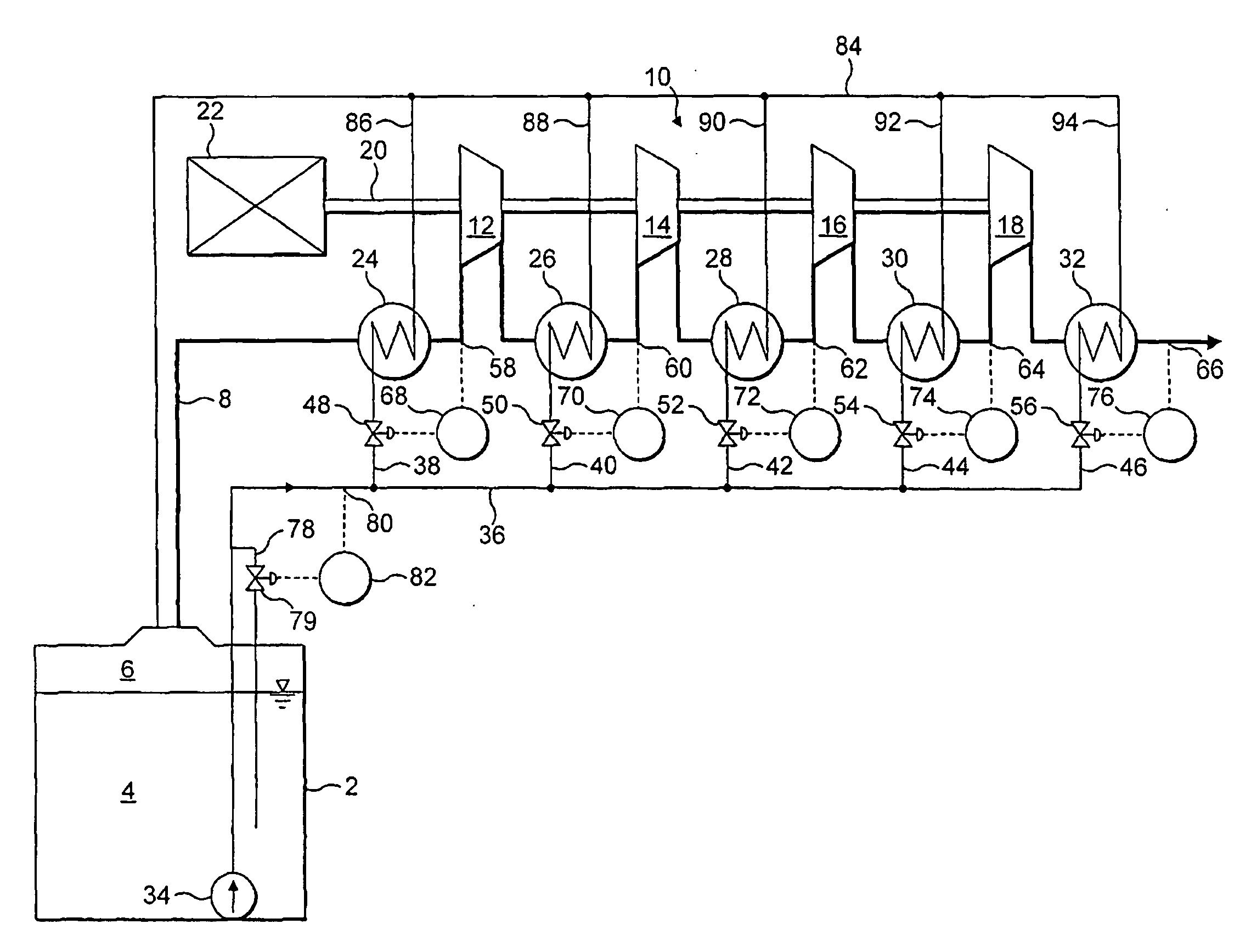
Compressor
InactiveUS20080008602A1Reduce consumptionSmall sizeAuxillariesContainer filling methodsEngineeringControl valves
A rotary liquefied natural gas boil-off compressor has a series of compression stages. A gas passage passes through the series of compression stages. The gas passage extends through and is in heat exchange relationship with cooling means in the form of indirect heat exchangers. Each of the heat exchangers is cooled by LNG supplied from a pipeline. Flow control valves are provided for controlling the flow of LNG to the heat exchangers respectively. The valves are controlled in response to temperature sensors respectively, so as to maintain the inlet temperature of each of the compression stages at a chosen sub-ambient temperature or between chosen sub-ambient temperature limits.
Owner:THE BOC GRP PLC
Breast pump system and methods
ActiveUS20160206794A1Prevents milk spillagePrevent backflowMilking pumpBlood pumpsMedicinePositive pressure
Systems and methods for pumping milk from a breast, wherein the milk is expressed from the breast under suction and milk is expulsed from the pumping mechanism to a collection container under positive pressure.
Owner:WILLOW INNOVATIONS INC
Image data compression employing multiple compression code tables
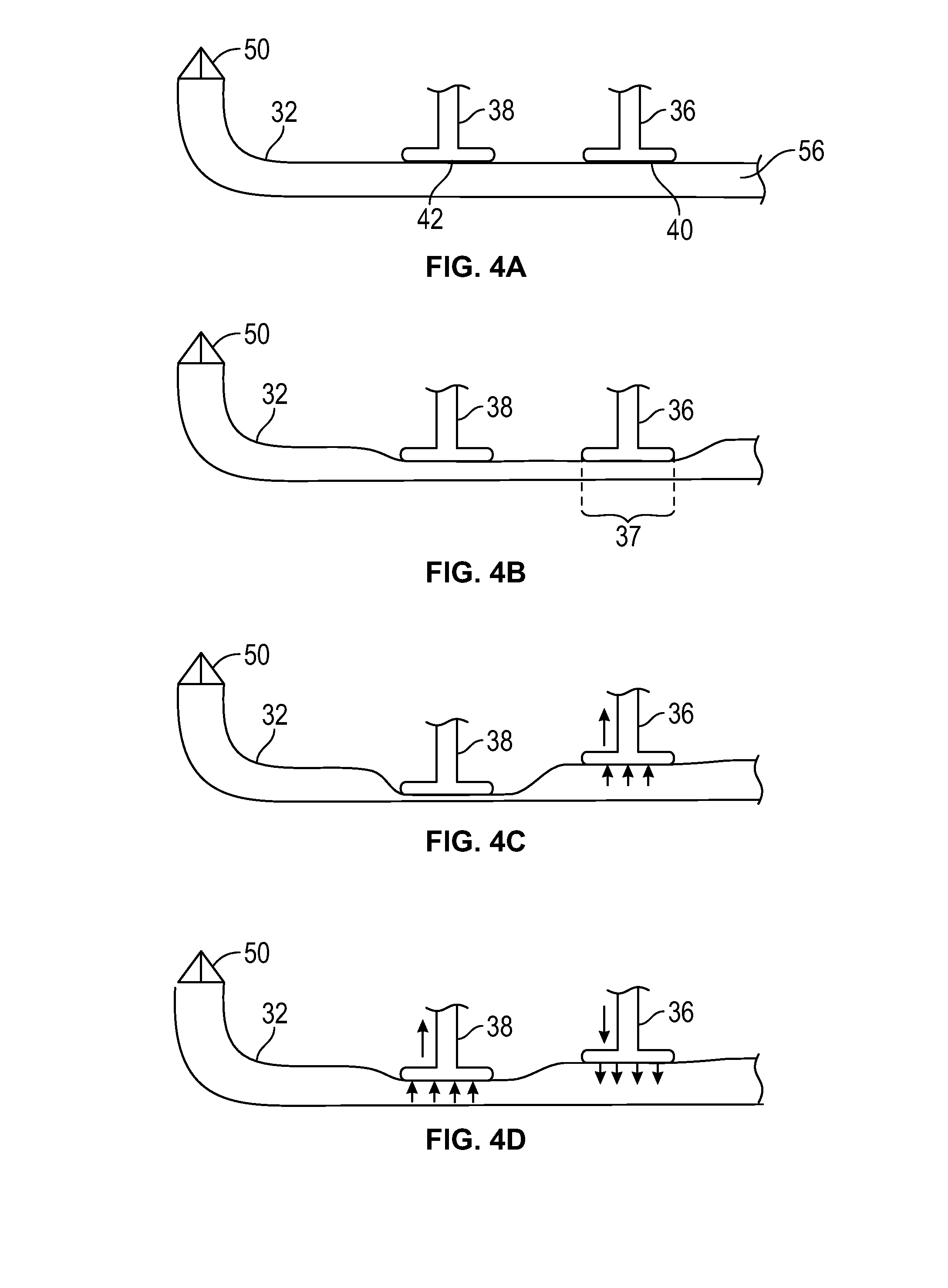
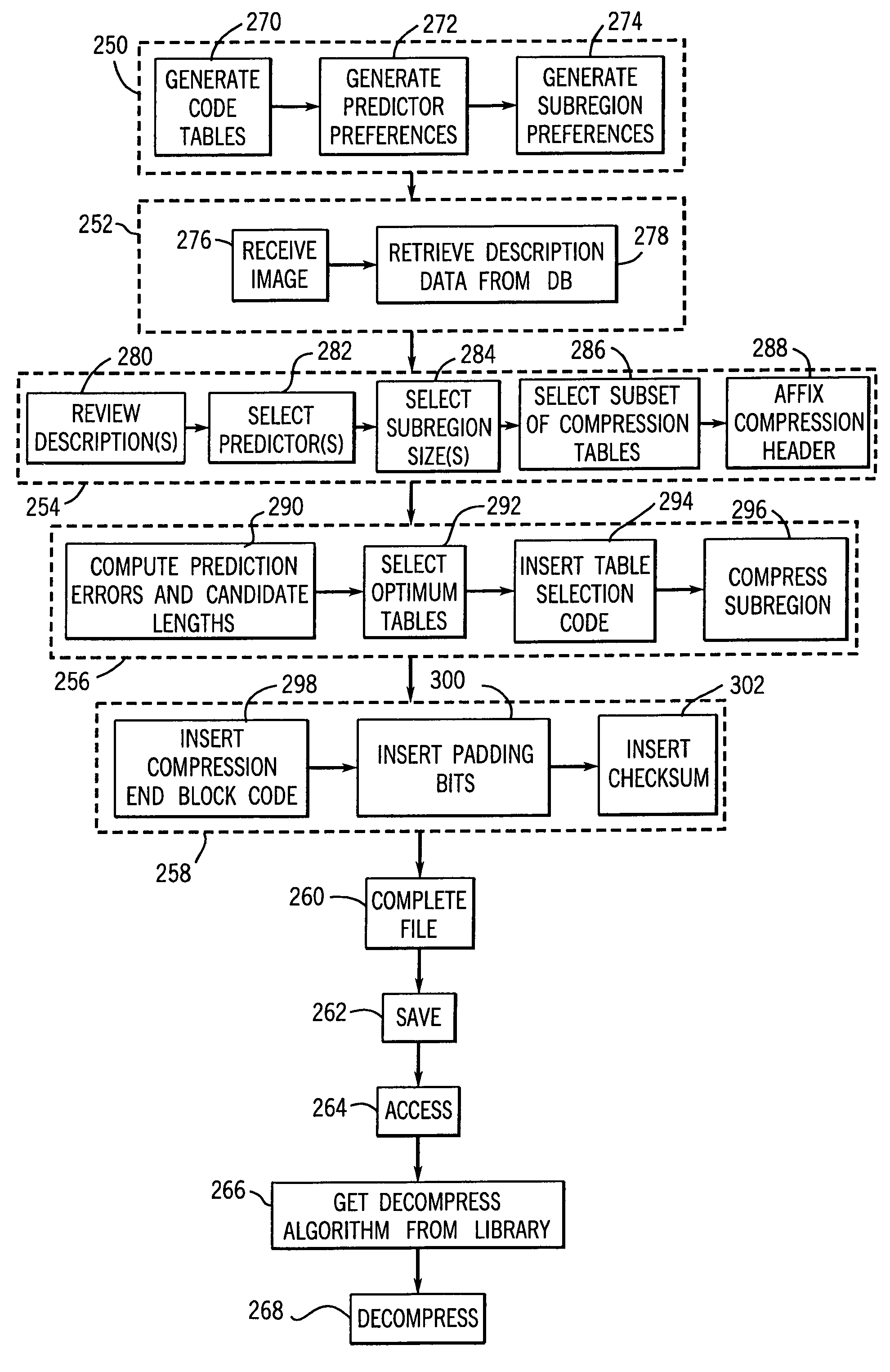
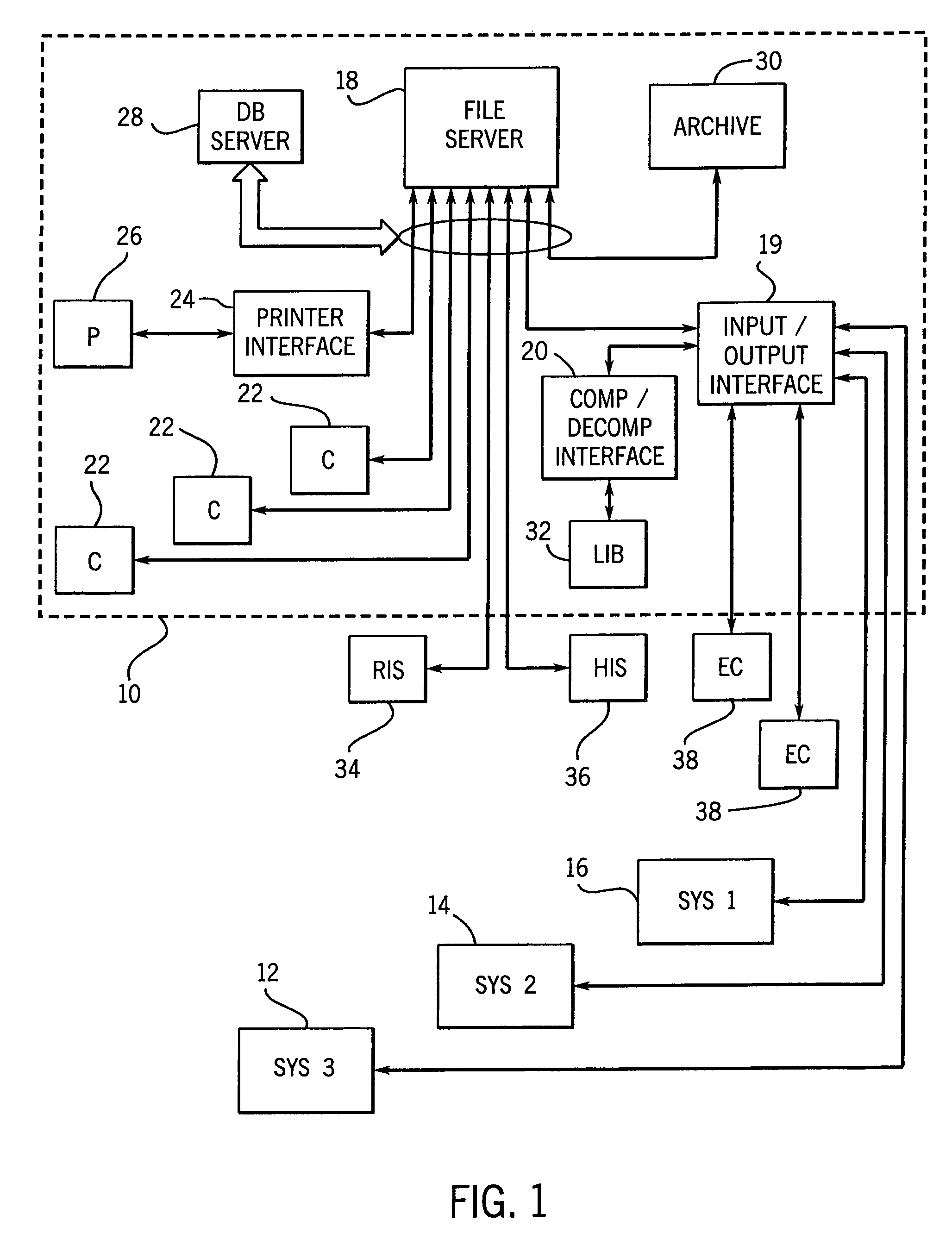
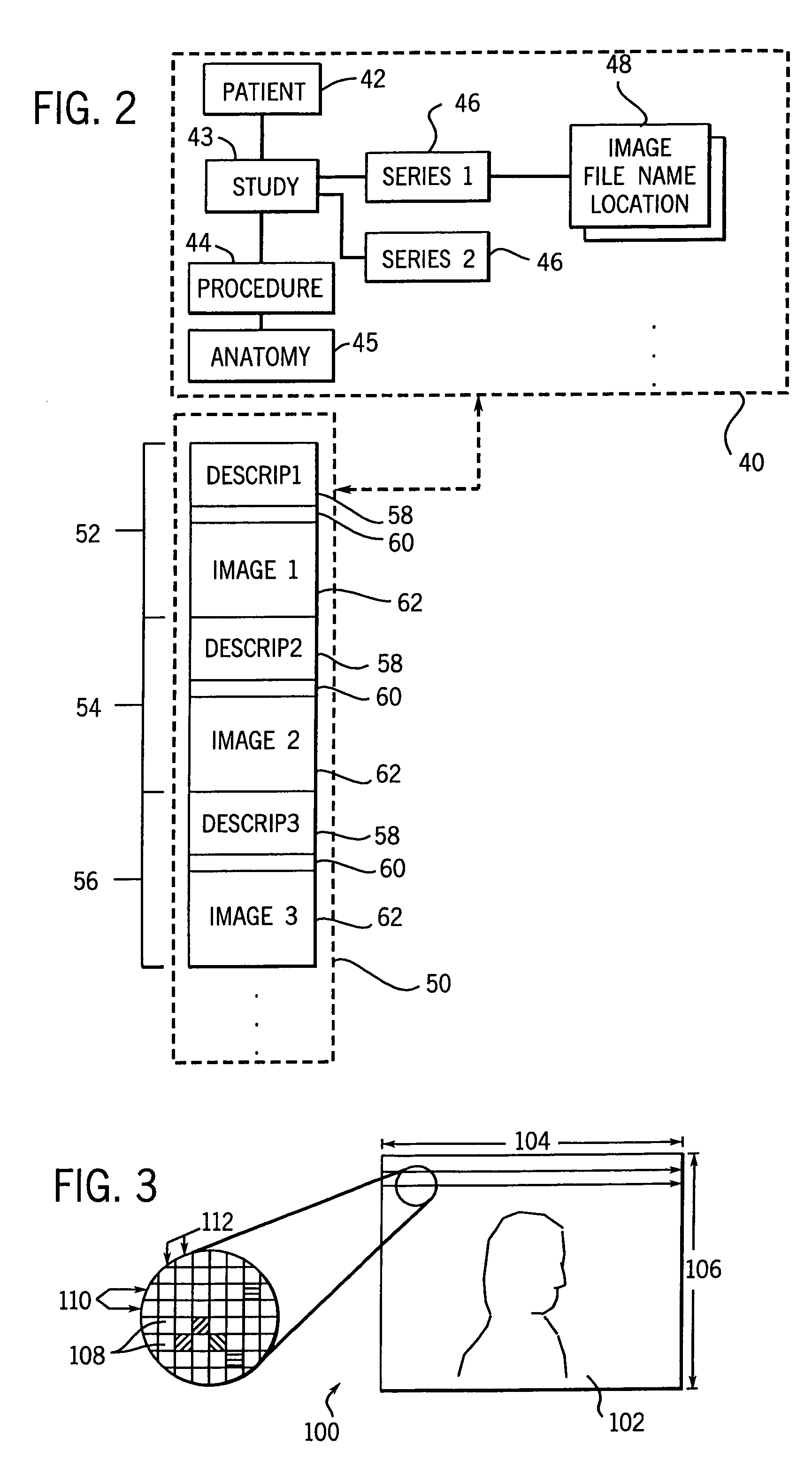
InactiveUS7050639B1Reduce compressionIncrease the compression ratioCharacter and pattern recognitionDigital video signal modificationData compressionData stream
An image data compression and decompression technique applies one or more compression code tables to optimally compress an image data stream. The compression code tables are established in accordance with anticipated image characteristics, and to accommodate different levels of variation or entropy in the image data. The image data may be divided into blocks or subregions for analysis of which of the candidate compression code tables provides the optimal compression of each subregion. The appropriate code table is selected for each subregion. The evaluation of the compression performance based upon application of each compression code table may include analysis of prediction differences or errors between predicted values for pixels of an image and the actual values for the pixels.
Owner:GENERAL ELECTRIC CO
Absorbent article
InactiveUS8237012B2Easy to separateReduce widthSanitary towelsBaby linensMechanical engineeringEngineering
Owner:UNI CHARM CORP
Synthetic resin container closure
InactiveUS20040060893A1Reduce compressionReduce injectionCapsClosure using stoppersEngineeringSynthetic resin
A container closure formed from a synthetic resin as a single unit has a circular top panel wall and a cylindrical skirt wall extending downwardly from the peripheral edge of the top panel wall. An outer cylindrical sealing protrusion, an inner cylindrical sealing protrusion, and annular sealing ridge, all having a predetermined shape and a predetermined size, are formed on the inner surface of the top panel wall. In one embodiment, the thickness of the center portion of the top panel wall is reduced to a predetermined range and a plurality of ribs having a predetermined thickness are formed on the inner surface of the center portion of the top panel wall.
Owner:JAPAN CROWN CORK CO LTD
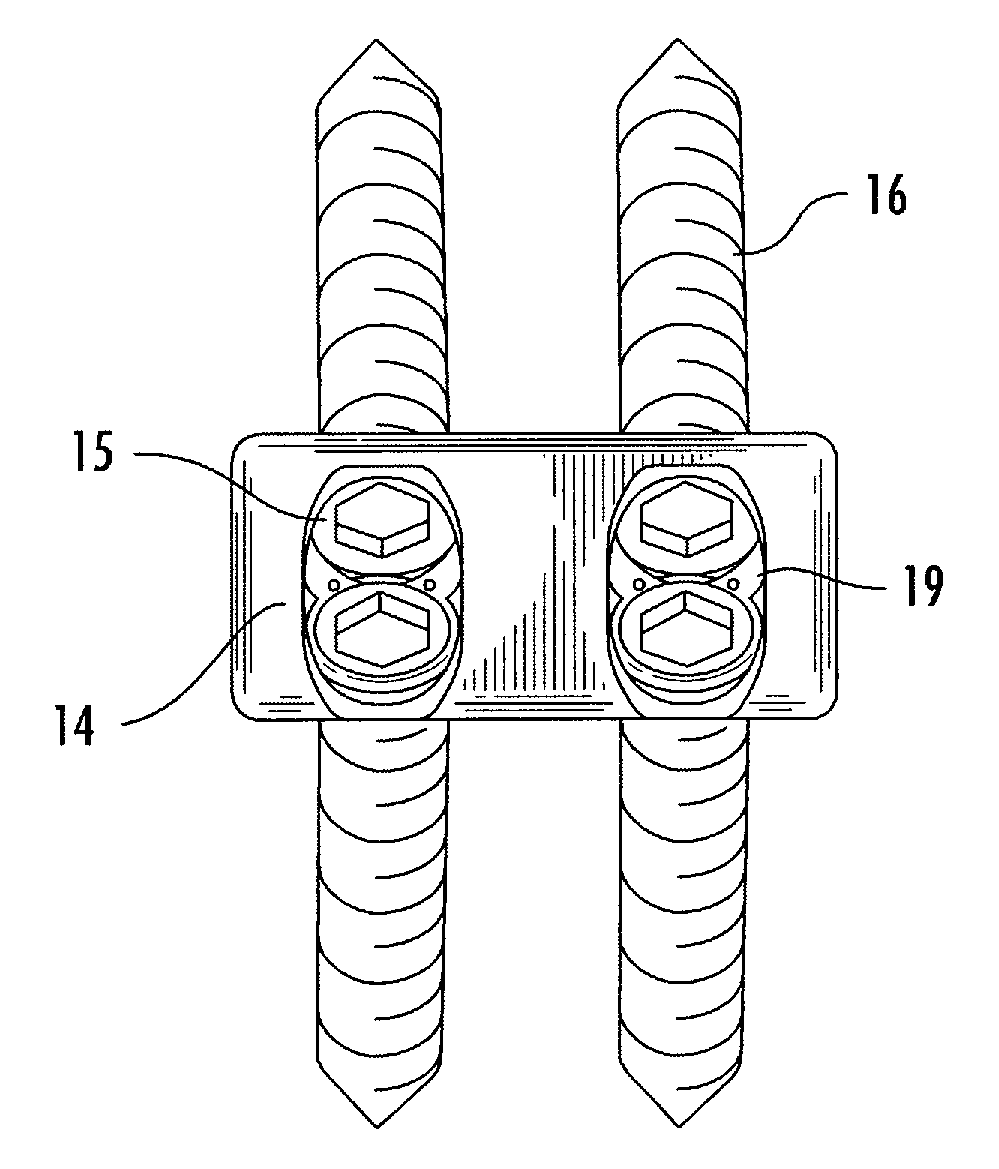
Retrieval Method For Opposed Slip Type Packers
ActiveUS20110147013A1Reduce compressionReduce sealFluid removalSealing/packingEngineeringMechanical engineering
A method is provided which releases and retrieves an opposed slip downhole tool by reducing the compressive forces on the sealing elements prior to unsetting the slip assemblies. Further, the method does so without damaging the slip assemblies. The method provides for the retrieval of the entire downhole tool including all of its component parts, requiring but a single trip within the wellbore. When the tool is to be retrieved, the sealing element is disengaged from the casing by relaxing the compression forces on the sealing element. Then the slip assemblies are disengaged from the casing such that the slip assemblies are no longer in gripping engagement with the casing. The tool is then retrieved from the wellbore. The step of disengaging the sealing assembly can be performed by radially contracting the sealing element with or without longitudinally expanding the sealing element.
Owner:HALLIBURTON ENERGY SERVICES INC
Non-invasive and minimally invasive and tightly targeted minimally invasive therapy methods and devices for parathyroid treatment
InactiveUS20130261368A1Effective regulationEffective treatmentElectrotherapyDiagnosticsNon invasiveTreatment system
Systems and method for treating organic tissue of a patient's body in precisely controlled regions of the body such as the parathyroid glands. The systems and methods include introducing an energy or substance into contact with target tissue to control functionality of the target tissue and preventing the energy or substance from contacting surrounding non-target tissues sufficiently that functionality of critical non-target tissues is substantially not affected.
Owner:SCHWARTZ ALAN N
Accommodating Intraocular Lenses
ActiveUS20110282442A1Speed up the conversion processEasy to adjustIntraocular lensIntraocular lensOptical power
An accommodating intraocular lens includes an optic portion a haptic portion and a backstop. The optic portion of the lens includes an actuator that deflects a lens element to alter the optical power of the lens responsive to forces applied to the haptic portion of the lens by contraction of the ciliary muscles. Forces applied to the haptic portion may result in fluid displacements from or to the haptic portion from the actuator. The backstop provides support to the haptic so that bulk translation of the haptic is prevented in response to the forces applied by the capsular sac.
Owner:ALCON INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com