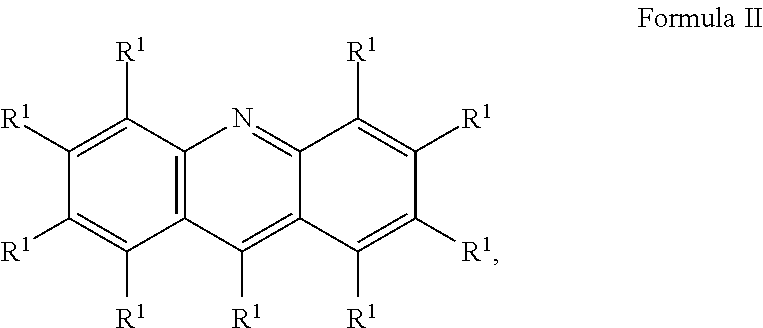
Liquid protein formulations containing water soluble organic dyes
a liquid protein and organic dye technology, applied in the field of injectable low viscosity pharmaceutical formulations, can solve the problems of difficulty in achieving chemical and/or physical stability, pain at the site, and difficulty in manufacturing, storage, etc., to facilitate and/or accelerate the reconstitution of the lyophilized dosage unit, reduce the viscosity of the protein solution, and facilitate processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Water Soluble Organic Dyes Lower the Viscosity of Concentrated Aqueous Solutions of High-Molecular-Weight Proteins
[0257]A commercially-obtained biosimilar AVASTIN® (100-400 mg) containing pharmaceutical excipients (Polysorbate 20, phosphate and citrate buffers, mannitol, and NaCl) was purified. First, Polysorbate 20 was removed using DETERGENT-OUT® TWEEN Medi Columns (G-Biosciences). Next, the resulting solutions were extensively buffer-exchanged into 20 mM sodium phosphate buffer (PB; pH 7.0) for PB samples and 2 mM PB (pH 7.0) for water soluble dye samples and concentrated to a final volume of less than 10 mL on Jumbosep centrifugal concentrators (Pall Corp.). Samples buffer exchanged into 2 mM PB were first aliquoted. Then, an appropriate amount of water soluble organic dye solution (pH 7.0) was added to each aliquot such that upon reconstitution with water, the final excipient concentration is 0.03-0.1 M. The protein solutions were then freeze-dried. The dried protein cakes, con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com