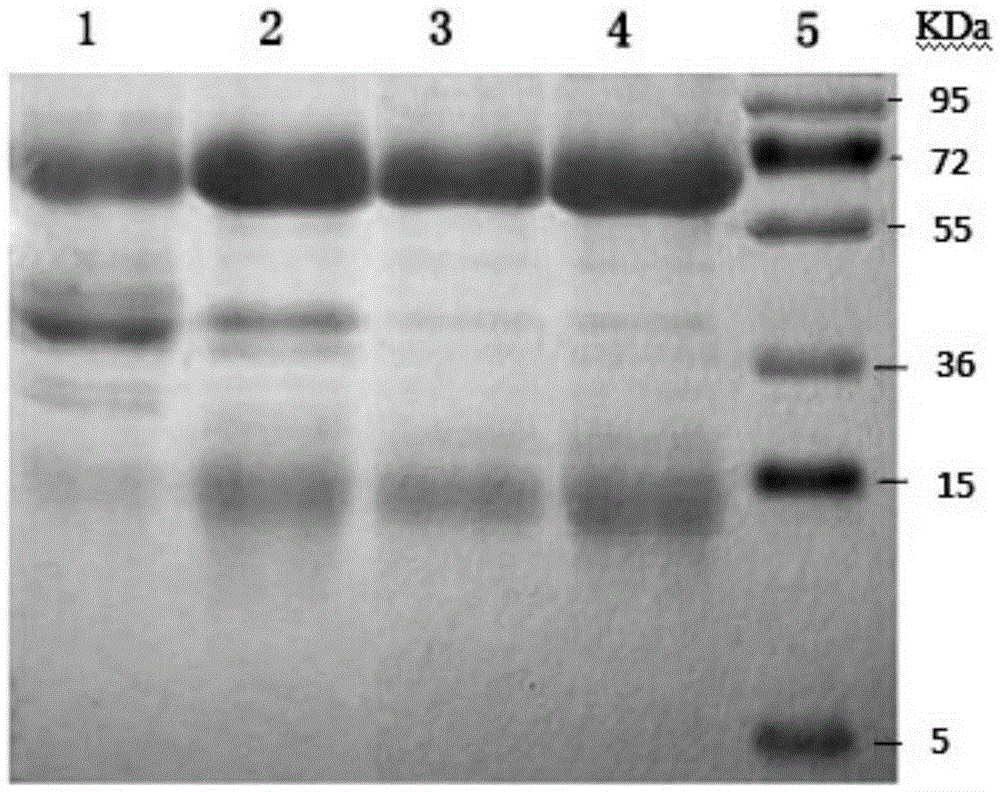
Hand-foot-and-mouth disease resistant specific nanobody and titer determination method thereof
A nano-antibody and hand, foot and mouth disease technology, applied in the field of immunology, can solve the problems of side effects, poor specificity, and low purity, and achieve the effect of good water solubility, small molecular weight, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of anti-hand, foot and mouth disease nanobody
[0042] 1. Antigen preparation
[0043] 1) Inoculate the EV71 virus liquid into the RD (strided muscle cell) cells grown to a monolayer, add 0.5mL to each bottle, put in CO 2 Cultivate in the incubator until about 90% of the cells appear CPE (cytopathic) and harvest, freeze and thaw 3 times at -20°C, centrifuge at 8000rpm at 4°C for 1h, collect the supernatant (virus liquid), centrifuge at 10000rpm at 4°C for 2h, discard clear, and the pellet was suspended in PBS buffer.
[0044] 2) CsCl density gradient centrifugation to purify virus antigen
[0045] Weigh 3.2g of CsCl into a 7.6mL gradient centrifuge tube, pipette gently until dissolved, centrifuge at 15000rpm at 4°C for 12h, and store at 4°C overnight. The next day, centrifuge at 20,000 rpm at 4°C for 4 hours to remove residual CsCl, discard the supernatant, add 300 μL PBS to dilute the purified virus solution and store at 4°C.
[0046] 2. Immunity
[0047...
Embodiment 2
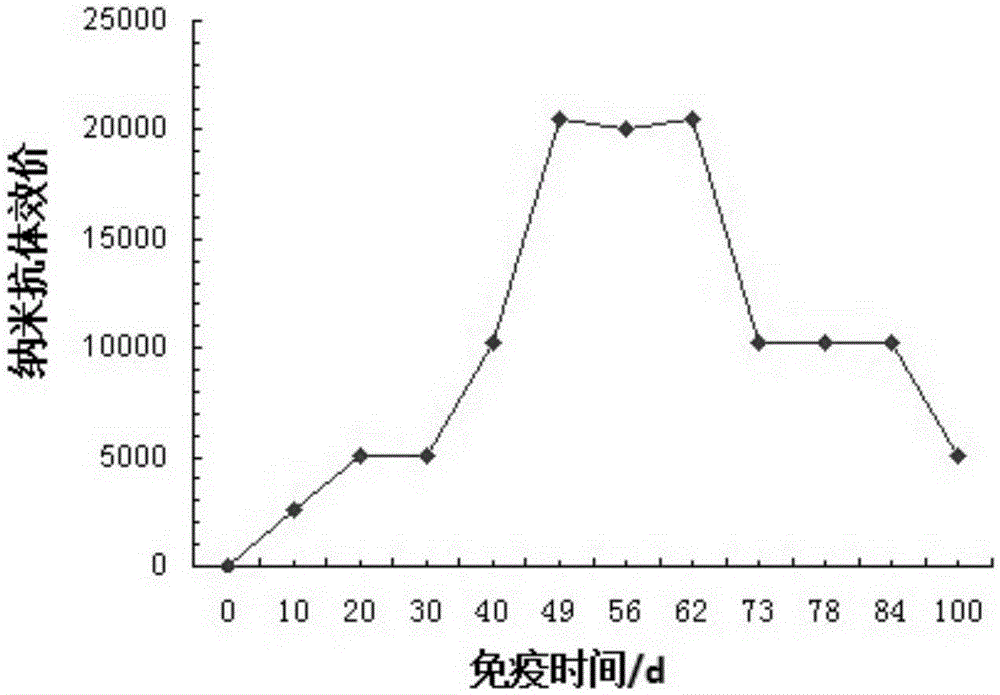
[0053] Antibody Titer Detection of Antibody in Camel Serum Against Hand, Foot and Mouth Disease
[0054] ELISA (enzyme-linked immunosorbent assay) method is used to detect the potency of the anti-hand, foot and mouth disease nanobody of the present invention. The test results showed that the titer of the anti-hand, foot and mouth disease nanobody reached 1:20480. see results figure 2 .
[0055] Potency determination procedure
[0056] 1) Coating: Dilute the purified EV71 virus antigen with 0.01MPBS1:1000 to the optimal coating solubility and coat it on a 96-well microtiter plate (100μL / well), incubate at 37°C for 1h, and then Overnight, the next day, the liquid in the wells was shaken dry, washed once with PBST, and patted dry with absorbent paper;
[0057] 2) Blocking: block with 0.01 MPBS solution containing 3% skimmed milk powder (200 μL / well), incubate at 37°C for 1 hour, spin dry, wash with PBST 3 times, each time for 2-5 minutes, and pat dry with absorbent paper;
...
Embodiment 3
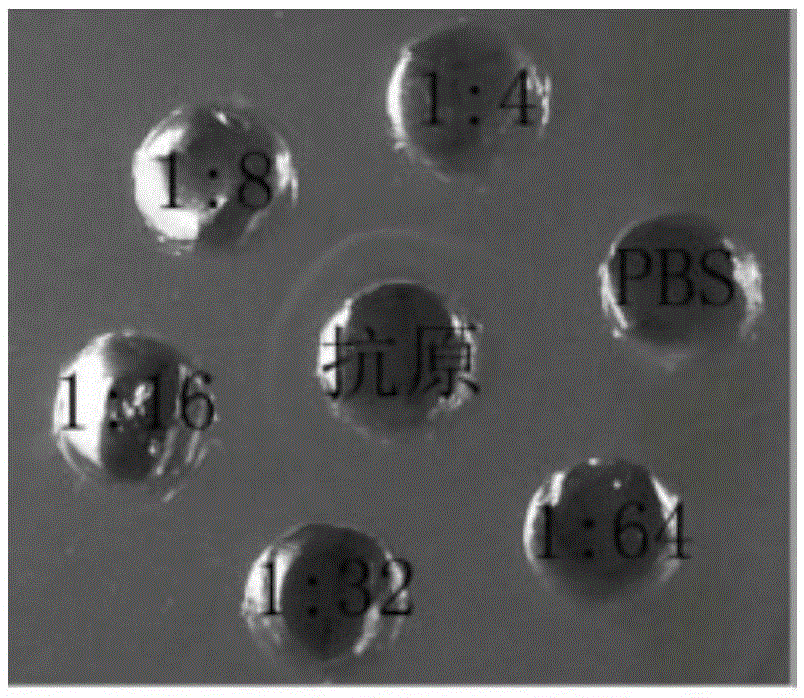
[0062] Immunobidirectional agar diffusion assay
[0063] Use a puncher to punch quincunx-like holes in the solidified agar culture dish, add EV71 virus antigen stock solution to the central hole of the quincunx hole, and add the same amount of different dilutions (1:4, 1:8, 1:16, 1:1) to the peripheral holes. 32, 1:64 and blank well PBS), the results are shown in image 3 , when the antibody was diluted at 1:4, 1:8, and 1:16, a significant white precipitation line appeared between the central antigen well and the nanobody well, but no precipitation line appeared between the blank control well PBS and the antigen well, which indicated that the prepared The nanobody has the ability to specifically bind to the EV71 antigen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com