Method for constructing pemphigus vulgaris animal model
A pemphigus vulgaris and construction method technology, applied in animal husbandry and other fields, can solve problems such as high cost, limited application of immunotherapy, and no PV animal model.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
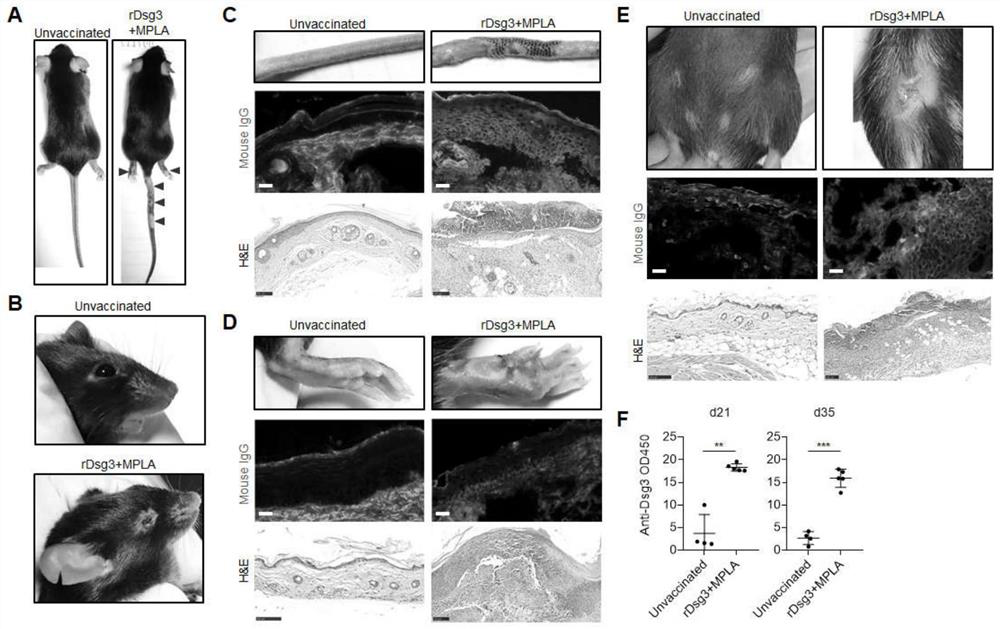
[0038] Example 1 MPLA as an immune adjuvant recombinant protein vaccine induces a PV model.
[0039] 1. Experimental materials
[0040] SPF grade 7-week female C57BL / 6J mice, recombinant Dsg3 protein solution (Nanjing Mingyan), MPLA (invivogen), 4% paraformaldehyde (Biosharp), OCT embedding medium (Sakura), goat anti-mouse IgG-FITC, Goat anti-mouse IgG-HRP (Abclonal), etc.
[0041] 2. Experimental method
[0042] 1. Vaccination of mice
[0043] Prepare recombinant protein vaccine. After the mice were anesthetized, 50 μl of recombinant protein vaccine (containing 5 μg of Dsg3 protein and 5 μg of MPLA) was subcutaneously inoculated through the soles of the feet for 3 consecutive days. On the 14th day, the mice were boosted and vaccinated in the same way for 3 days.
[0044] 2. Rat tail, sole and back skin tissue extraction and histological examination
[0045] On day 35, mice were sacrificed by cervical dislocation after anesthesia. Use a sharp scalpel and ophthalmic scis...
Embodiment 2
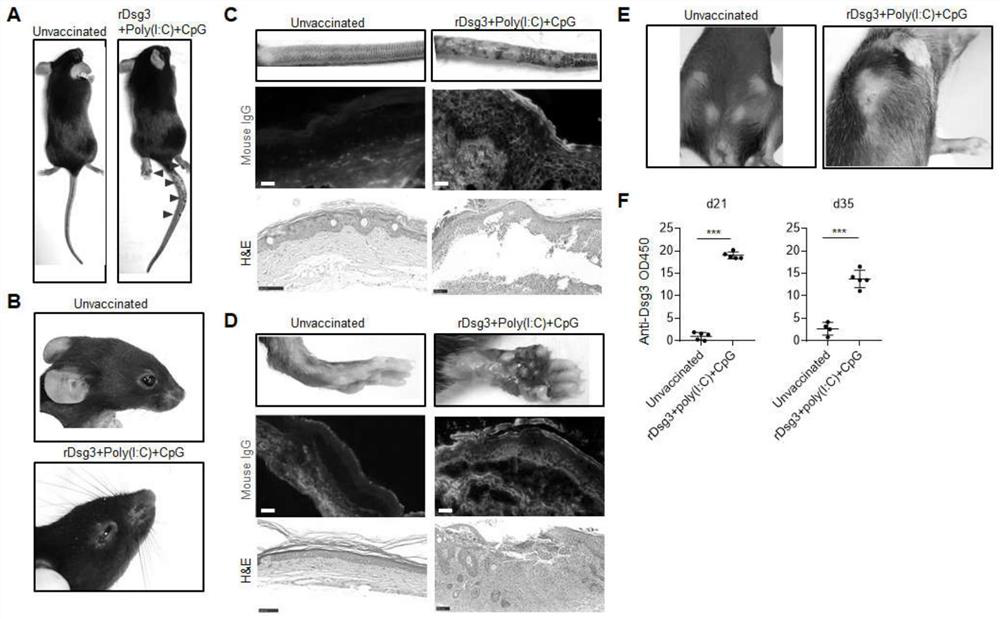
[0052] Example 2 Poly(I:C) and CpG1826 as immune adjuvant recombinant protein vaccine induced PV model.
[0053] 1. Experimental materials
[0054] SPF grade 7-week female C57BL / 6J mice, recombinant Dsg3 protein solution (Nanjing Mingyan), poly(I:C) (invivogen), CpG1826 (Sangong), 4% paraformaldehyde (Biosharp), OCT embedding medium (Sakura), goat anti-mouse IgG-FITC, goat anti-mouse IgG-HRP (Abclonal), etc.
[0055] 2. Experimental method
[0056] 1. Vaccination of mice
[0057] Recombinant protein vaccine was prepared. After anesthesia, mice were subcutaneously inoculated with 50 μl recombinant protein vaccine (including Dsg3 protein 5 μg, poly(I:C) 30 μg, and CpG1826 1 μg) for 3 consecutive days. On the 14th day, mice were boosted and vaccinated in the same way for 3 days.
[0058] 2. Rat tail, sole, back skin tissue extraction and histological examination
[0059] On day 35, mice were sacrificed by cervical dislocation after anesthesia. Use a sharp scalpel and ophtha...
Embodiment 3
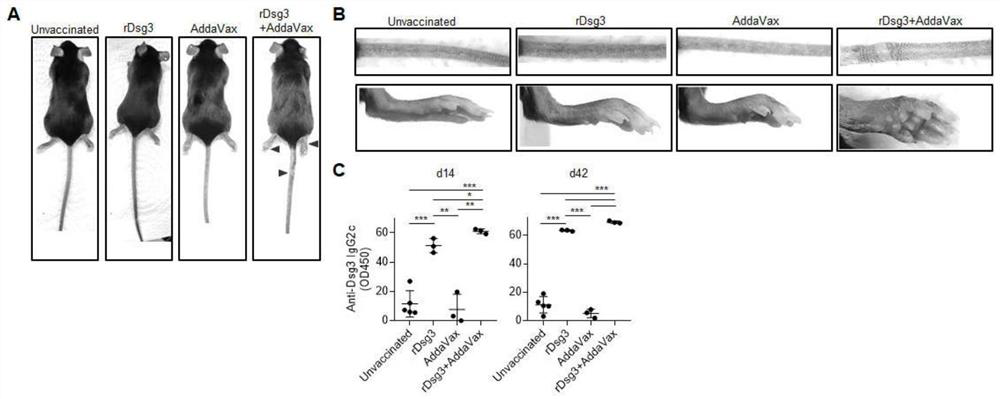
[0066] Example 3 AddaVax is an immune adjuvant recombinant protein vaccine induced PV model.
[0067] 1. Experimental materials
[0068] SPF grade 7-week female C57BL / 6J mice, recombinant Dsg3 protein solution (Nanjing Mingyan), AddaVax adjuvant (invivogen), goat anti-mouse IgG2c-HRP (Southern Biotech), etc.
[0069] 2. Experimental method
[0070] 1. Vaccination of mice
[0071] The recombinant protein vaccine was prepared according to the ratio of Dsg3 protein and AddaVax volume to 1:1. After the mice were anesthetized, 50 μl of recombinant protein vaccine (containing 5 μg of Dsg3 protein and 25 μl of AddaVax) was subcutaneously inoculated through the soles of the feet for 3 consecutive days. On the 14th day, the mice were boosted and vaccinated in the same way for 3 days.
[0072] 2. ELISA assay
[0073] Serum was prepared at different time points, aliquoted and frozen for ELISA. Prepare 50 μg / ml Dsg3 solution, add 100 μl / well to the ELISA plate, coat overnight at 4°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com