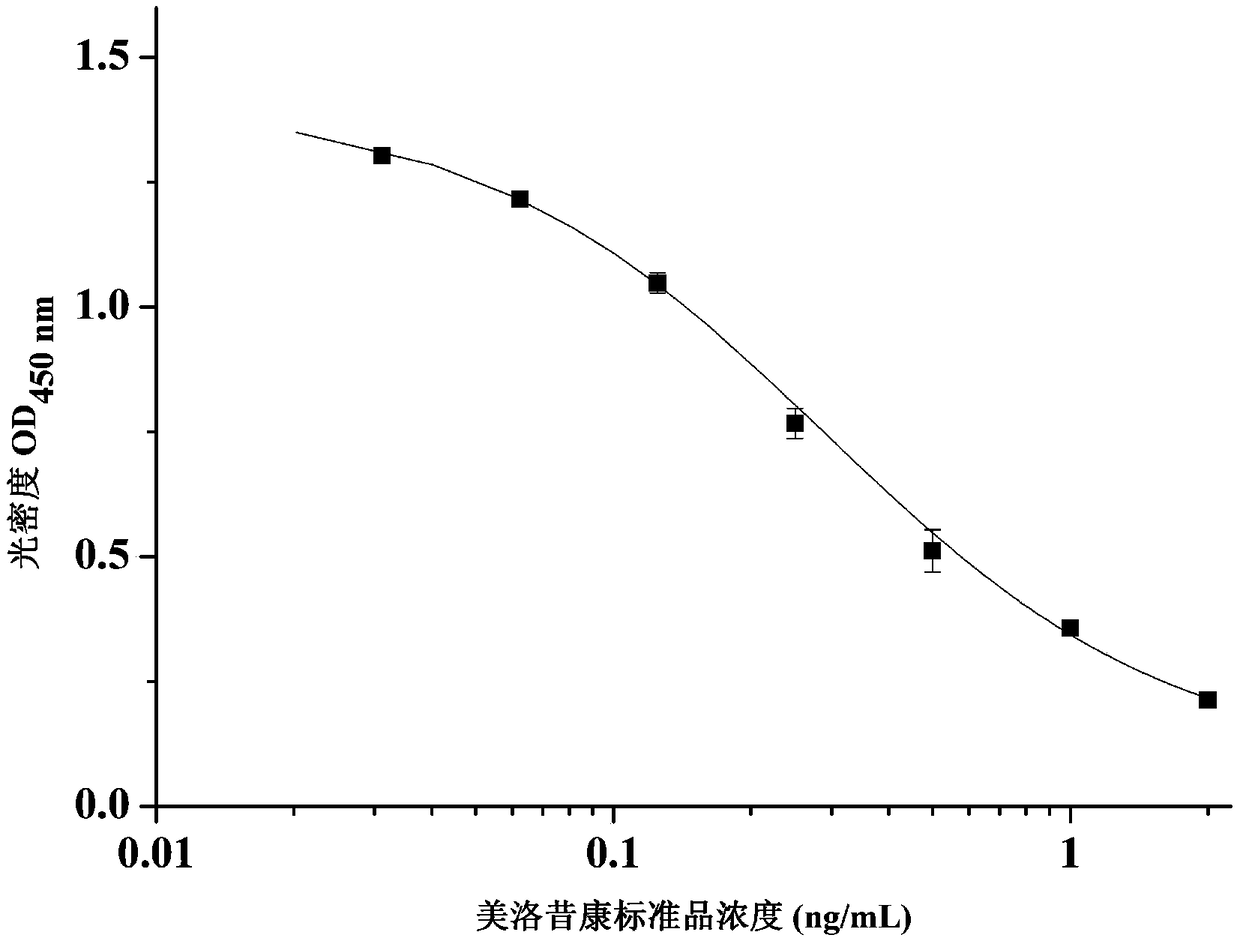
Hybridoma cell strain secreting meloxicam monoclonal antibody and application of hybridoma cell strain
A hybridoma cell line and monoclonal antibody technology, which is applied to cells modified by introducing foreign genetic material, fusion cells, hybrid peptides, etc., can solve the problems of time-consuming, expensive, and inability to realize rapid detection of a large number of samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: the synthesis of meloxicam hapten
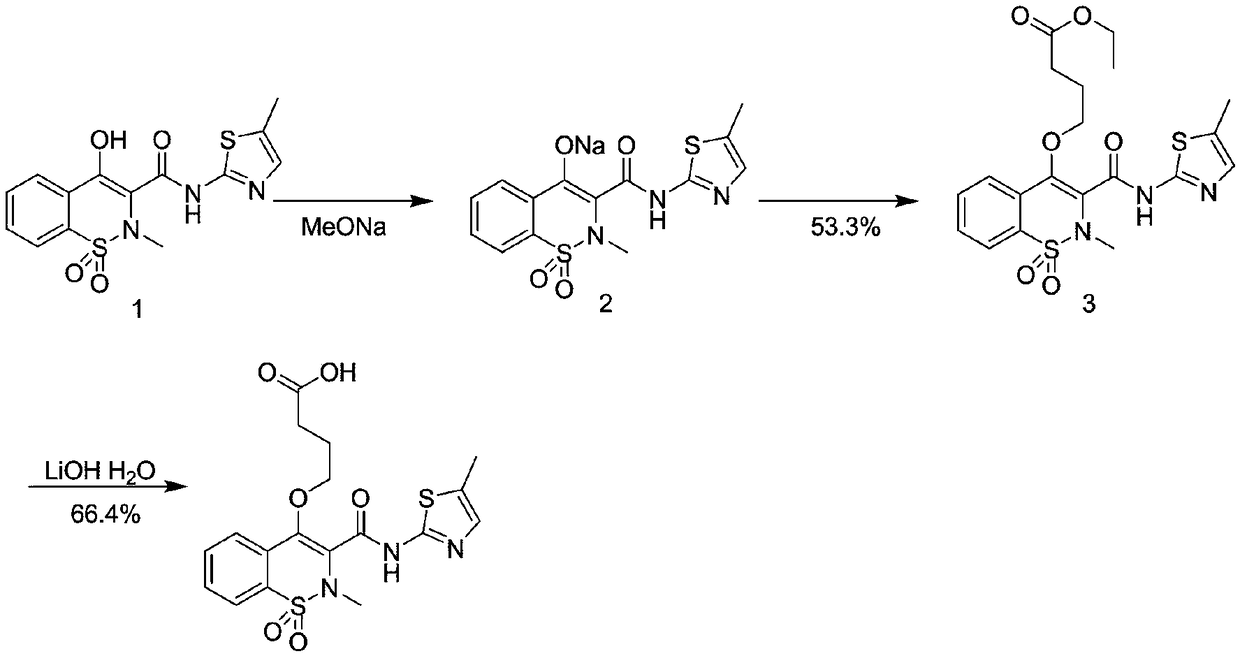
[0092] Synthetic routes such as figure 1 .
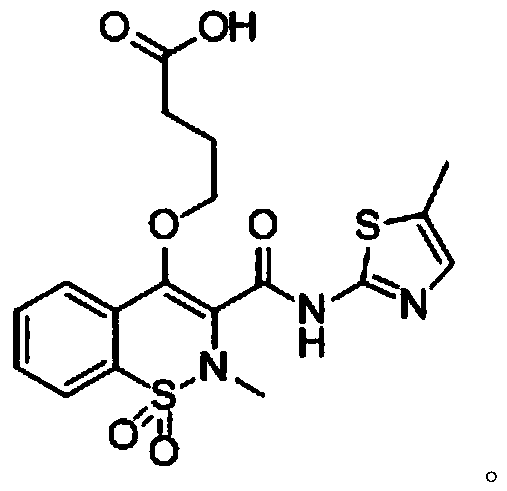
[0093]1g of compound 1 was dissolved in 50mL of methanol solution, and 157mg of MeONa was added and stirred overnight at 65°C to obtain mixture 1; the obtained mixture was concentrated to obtain compound 2; 1.20g of compound 2 was dissolved in 30mL of DMF, and 1.20mg of 4- Ethyl bromobutyrate was stirred overnight at 80°C to obtain a mixture 2; the step 4 was to concentrate the obtained mixture 2 and then purify it with a silica gel column to obtain compound 3; 800 mg of compound 3 was dissolved in 3 mL of tetrahydrofuran and 1 mL of water 180 mg of lithium hydroxide monohydrate was added to the mixed solution to adjust the pH to 4-6, and stirred at room temperature overnight to obtain a mixture 4; the aqueous layer of the mixture 4 was extracted with ethylamine, and the organic layers were combined, washed with brine, and dried over sodium sulfate. Concentrate to obtain mel...
Embodiment 2
[0096] Embodiment 2: the synthesis of complete antigen of meloxicam
[0097] Dissolve 1.7 mg of meloxicam hapten (Melo), 2.2 mg of 1-ethylcarbodiimide hydrochloride, 1.4 mg of N-hydroxysuccinimide in 400 μL of anhydrous N,N-dimethylformamide , stirred at room temperature for 6-8 hours to obtain A1 solution; take 10 mg of keyhole limpet hemocyanin (KLH) (the molar ratio of Melo to KLH is 1500:1) and dilute it with an appropriate amount of boric acid buffer solution to obtain B1 solution; at room temperature, dropwise Add solution A1 to solution B1, react overnight at room temperature to obtain a mixed solution; separate the complete antigen and uncoupled small molecule hapten (Melo) from the mixed solution by dialysis to obtain the complete antigen (Melo-KLH).
[0098] Dissolve 1.7 mg of meloxicam hapten (Melo), 2.2 mg of 1-ethylcarbodiimide hydrochloride, 1.4 mg of N-hydroxysuccinimide in 400 μL of anhydrous N,N-dimethylformamide , and stirred at room temperature for 6-8h to ...
Embodiment 3
[0100] Embodiment 3: the synthesis of meloxicam coating former
[0101] Dissolve 1.8 mg of meloxicam hapten (Melo), 2.4 mg of 1-ethylcarbodiimide hydrochloride, and 1.45 mg of N-hydroxysuccinimide in 300 μL of anhydrous N,N-dimethylformamide, Stir at room temperature for 6-8 hours to obtain liquid A2; dissolve 5 mg of chicken ovalbumin (OVA) in 2 mL of boric acid buffer solution to obtain liquid B2; at room temperature, add liquid A2 dropwise to liquid B2, and react overnight at room temperature to obtain Mixed solution; the mixed solution was separated by dialysis from the complete antigen and the uncoupled small molecule hapten (Melo), and the coated original (Melo-OVA).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com