Solid pharmaceutical dosage forms comprising bisphosphonates and modified amino acid carriers
a technology of bisphosphonates and amino acids, applied in the field of pharmaceutical compositions, can solve the problems of limited delivery of active agents, and achieve the effect of facilitating absorption of bisphosphonates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
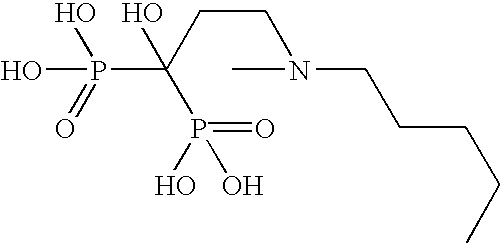
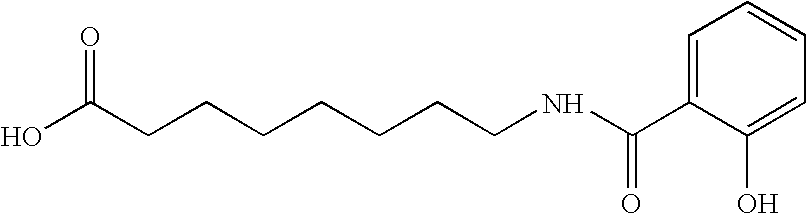
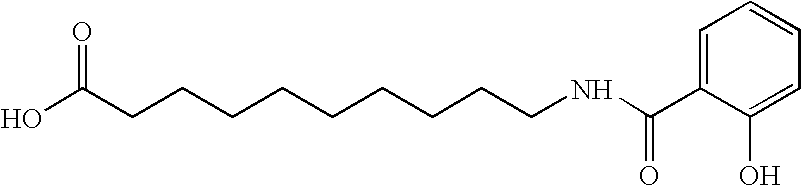
[0076] In this example, 30 mg film coated ibandronic acid tablets were prepared wherein the ratio of ibandronic acid to sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC) was 1:5.
Formulation CompositionItem#Ingredientmg per TabletKernel Composition1.Ibandronate Monosodium Salt Monohydrate33.7512.Sodium N-[8-(2-hydroxybenzoyl)amino]caprylate161.8123.Lactose Monohydrate (Pharmatose ® 350)17.504.Croscarmellose Sodium (Ac-Di-Sol ®)13.445.Polyvinylpyrrolidone (Povidone; PVP K30)30.506.Purified Water3q.s.7.Croscarmellose Sodium (Ac-Di-Sol ®) (External)9.258.Sodium Stearyl Fumarate (External)3.75Total Weight of Kernel270.00Coating Composition9.Opadry White YS-1-700205.5010.Purified Water3q.s.Total Weight of Film Coated Tablet275.50
1Equivalent to 30 mg of anhydrous ibandronic acid. The quantity of ibandronic acid is to be adjusted if the assay is less than 100%. If the assay adjustment is required, the quantity of Pharmatose ® 350 is reduced to compensate for the increase in ibandronic ...
example 2
[0080] In this example, 30 mg film coated ibandronic acid tablets were prepared wherein the ratio of ibandronic acid to sodium N-[8-(2-hydroxybenzoyl) amino]caprylate (SNAC) was 1:10.
Formulation CompositionItem#Ingredientmg per TabletKernel Composition1.Ibandronate Monosodium Salt Monohydrate33.7512.Sodium N-[8-(2-hydroxybenzoyl)amino]caprylate323.6323.Lactose Monohydrate (Pharmatose ® 350)35.004.Croscarmellose Sodium (Ac-Di-Sol ®)25.625.Polyvinylpyrrolidone (Povidone; PVP K30)61.006.Purified Water3q.s.7.Croscarmellose Sodium (Ac-Di-Sol ®) (External)18.508.Sodium Stearyl Fumarate (External)7.50Total Weight of Kernel505.00Coating Composition9.Opadry White YS-1-700210.5010.Purified Water3q.s.Total Weight of Film Coated Tablet515.50
1Equivalent to 30 mg of anhydrous ibandronic acid. The quantity of ibandronic acid is to be adjusted if the assay is less than 100%. If the assay adjustment is required, the quantity of Pharmatose ® 350 is reduced to compensate for the increase in ibandron...
example 3
[0084] In this example, 30 mg film coated ibandronic acid tablets were prepared wherein the ratio of ibandronic acid to sodium N-[8-(2-hydroxybenzoyl)amino] caprylate (SNAC) was 1:20.
Formulation CompositionItem#Ingredientmg per TabletKernel Composition1.Ibandronate Monosodium Salt Monohydrate33.7512.Sodium N-[8-(2-hydroxybenzoyl)amino]caprylate647.2523.Lactose Monohydrate (Pharmatose ® 350)70.004.Croscarmellose Sodium (Ac-Di-Sol ®)50.005.Polyvinylpyrrolidone (Povidone; PVP K30)122.006.Purified Water3q.s.7.Croscarmellose Sodium (Ac-Di-Sol ®) (External)37.008.Sodium Stearyl Fumarate (External)15.00Total Weight of Kernel975.00Coating Composition9.Opadry White YS-1-700220.0010.Purified Water3q.s.Total Weight of Film Coated Tablet995.00
1Equivalent to 30 mg of anhydrous ibandronic acid. The quantity of ibandronic acid is to be adjusted if the assay is less than 100%. If the assay adjustment is required, the quantity of Pharmatose ® 350 is reduced to compensate for the increase in ibandr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com