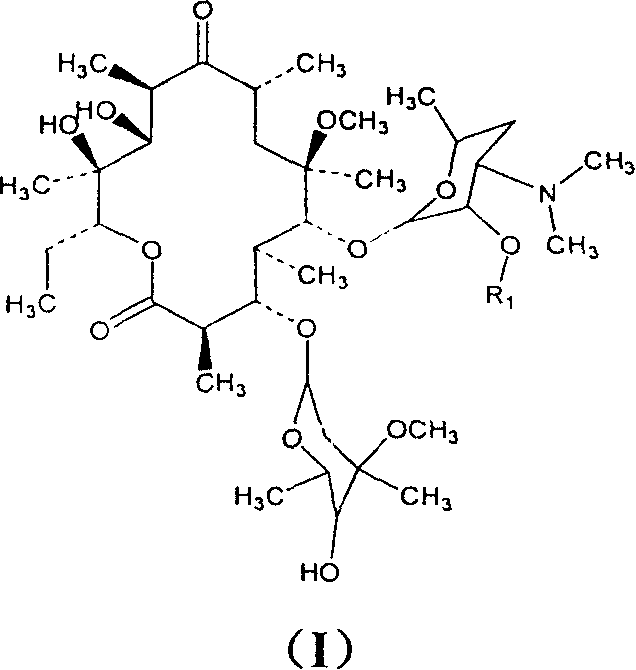
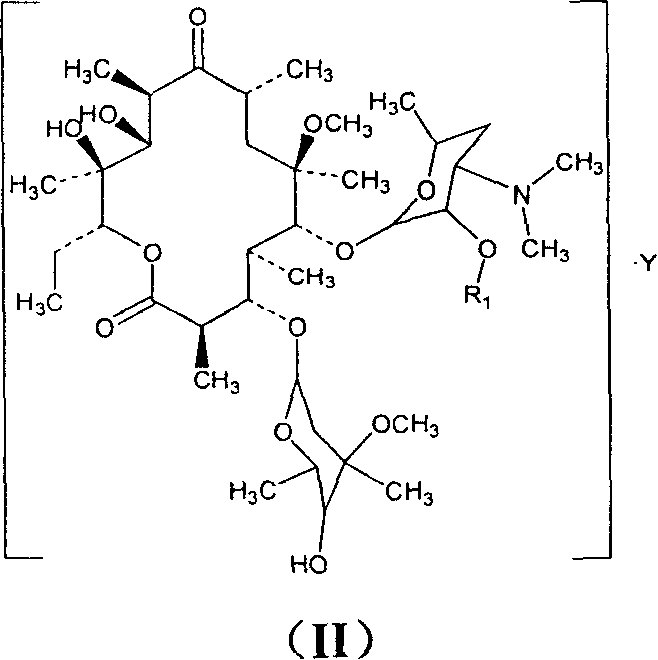
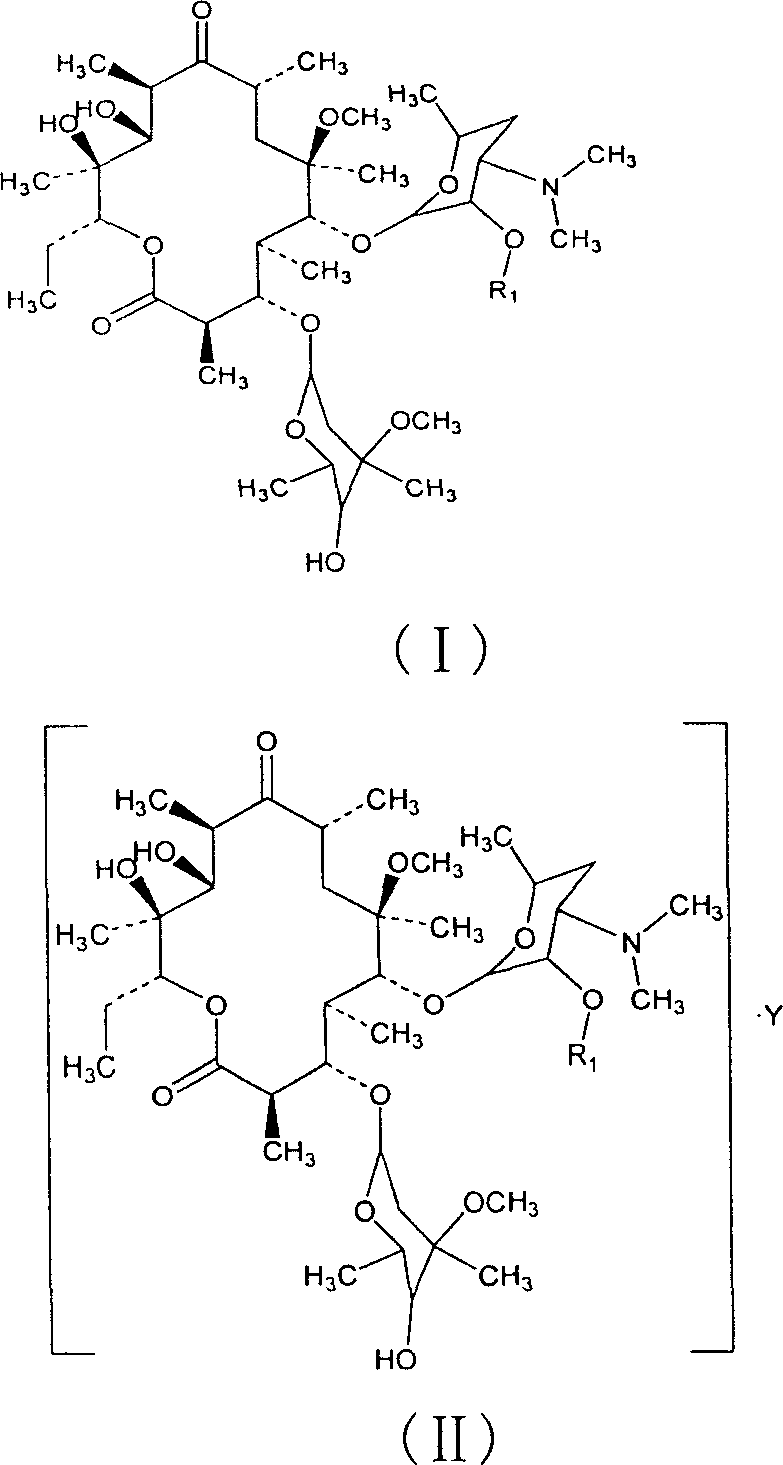
Clarithromycin derivatives and its preparation process and pharmaceutical application
A technology of clarithromycin and derivatives, applied in the field of derivatives of clarithromycin, can solve problems such as clarithromycin taste bitter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0110] Example 1 clarithromycin-2'-ethylsuccinate
[0111] method one
[0112] Clarithromycin 6.0g, dissolved in 20ml THF, 4.8g K 2 CO 3 A solution made of 22ml of water was added, the mixture was stirred and cooled to 5-10°C, 3.7g of succinic monoethyl chloride was added over 40 minutes, then stirred in an ice bath for 30 minutes, and then stirred overnight at room temperature . Add 0.3g of sodium citrate, adjust the pH value of the reaction aqueous solution between 8.0-9.0 with NaOH solution, stir for half an hour, the solution is layered, take the organic layer and add diatomaceous earth, filter, and the filtrate is cooled to 10- Add about 40ml of water at 15°C, a white solid precipitates out of the solution, filter, wash with cold acetone, and dry the filter cake in an oven at 50-60°C to obtain 6.2g of white solid with a yield of 88.3%.
[0113] The solid was recrystallized in acetone to obtain white crystals with a melting point of 217-222°C.
[0114] Method Two
[...
example 2
[0120] Example 2 clarithromycin-2'-propionate
[0121] method one
[0122] Clarithromycin 6.0g, dissolved in 20ml THF, 4.8g K 2 CO 3 A solution made of 22ml of water was added, the mixture was stirred and cooled to 5-10°C, 3.0g of propionyl chloride was added over 40 minutes, stirred in an ice bath for 30 minutes, and then stirred overnight at room temperature. Add 0.3g of sodium citrate, adjust the pH value of the reaction aqueous solution between 8.0-9.0 with NaOH solution, stir for half an hour, the solution is layered, take the organic layer and add diatomaceous earth, filter, and the filtrate is cooled to 10- Add about 40ml of water at 15°C, a white solid precipitates out of the solution, filter, wash with cold acetone, and dry the filter cake in an oven at 50-60°C to obtain 5.5g of white solid with a yield of 85.9%.
[0123] Method Two
[0124] Dissolve 6.0g of clarithromycin in 30ml of THF with stirring, add 5.0g of propionic anhydride, add dropwise 0.5ml of concent...
example 3
[0125] Example 3 clarithromycin-2'-isobutyrate
[0126] method one
[0127] Clarithromycin 6.0g, dissolved in 20ml THF, 4.8g K 2 CO 3 A solution made of 22ml of water was added, the mixture was stirred and cooled to 5-10°C, 5.0g of isobutyryl chloride was added over 40 minutes, stirred in an ice bath for 30 minutes, and then stirred overnight at room temperature. Add 0.3g of sodium citrate, use NaOH solution to adjust the pH value of the reaction aqueous solution between 8.0-9.0, stir for half an hour, the solution is layered, take the organic layer, concentrate to obtain a white solid, add ether to dissolve the solid, and heat Wash, cool and filter, and dry the filter cake in an oven at 30-40°C. The weight of the white solid is 5.0 g, and the yield is 76.2%.
[0128] Method Two
[0129]Dissolve 6.0g of clarithromycin in 30ml of THF with stirring, add 5.0g of isobutyric anhydride, dropwise add 0.5ml of concentrated sulfuric acid, stir and react for 3h, then add about 40ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com