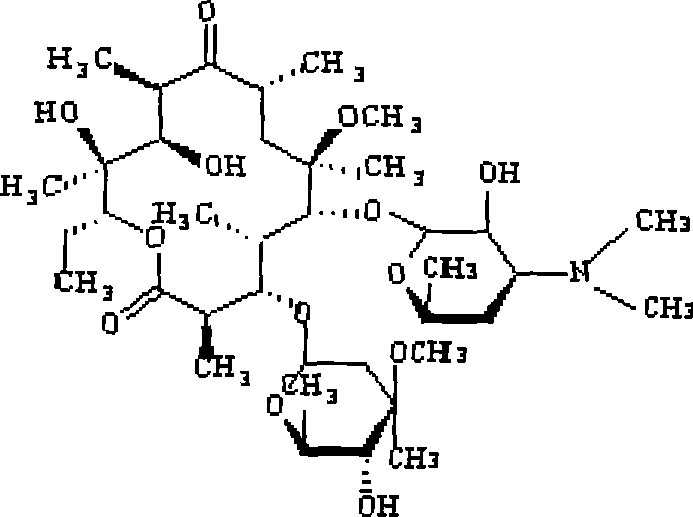
Method for preparing clarithromycin granule without bitter taste
A technology of clarithromycin and bitterness, which is applied in the field of preparation of clarithromycin drug pellets, can solve the problems of affecting curative effect, decreased compliance with doctor's orders, etc., and achieves the effects of good therapeutic effect, abundant dosage forms and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Preparation of clarithromycin debitter granules
[0033] (1) Preparation of coating solution:
[0034] Solution A: Take 100g of glyceryl monostearate, Tween-805g, 1200g of pure water, and stir well;
[0035] Solution B: Take Eudragit L30D55 5880g, triethyl citrate 176g, microcrystalline cellulose 100g, pure water 3700g, stir well.
[0036] Solution C: Take 60g of Eudragit E-100, 1140g of 95% ethanol, fully stir to dissolve,
[0037] Then, add pure water to solution A, solution B and solution C to 18520g, set aside.
[0038] (2) Coating
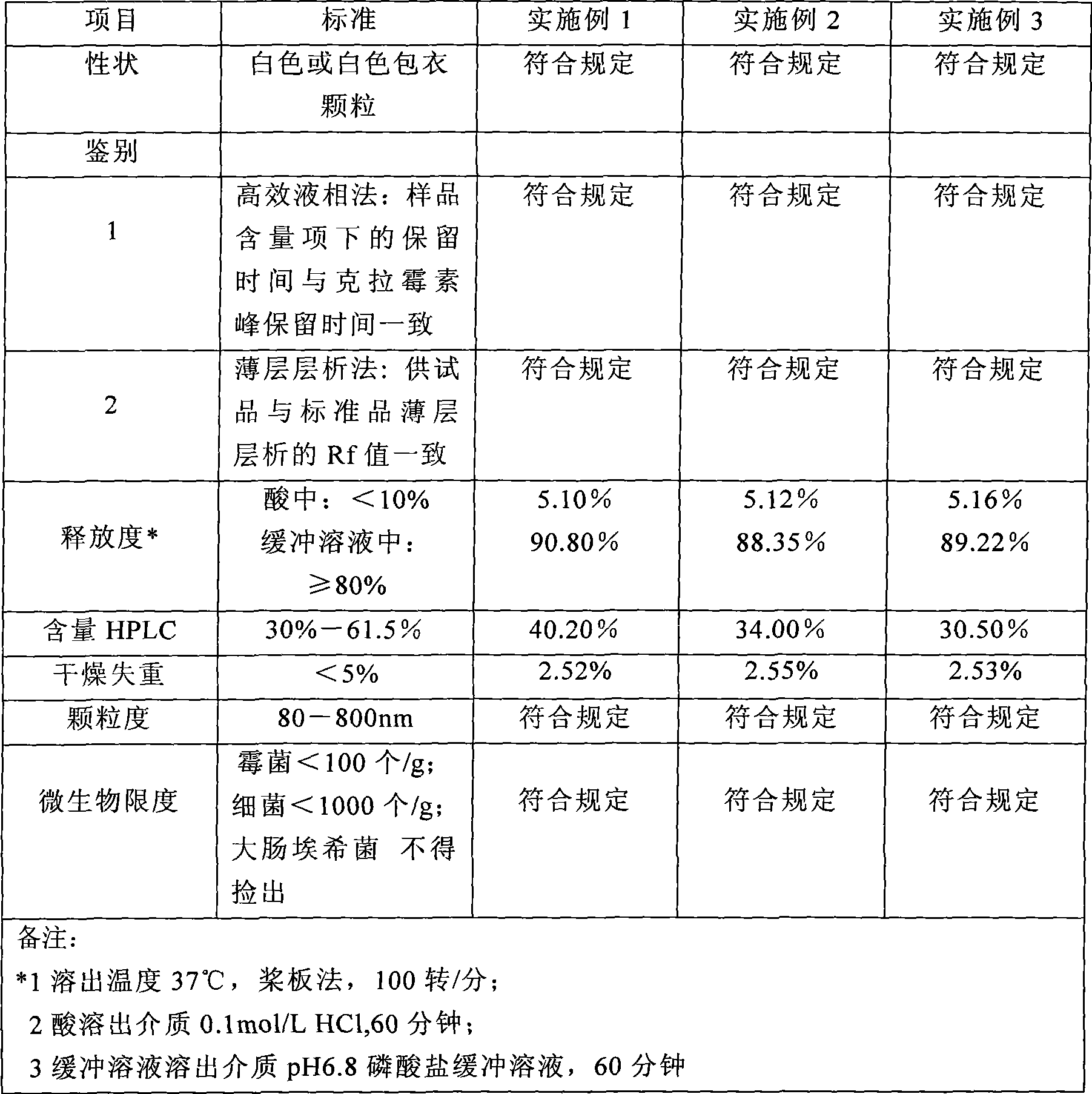
[0039] Take 2000g 80 mesh clarithromycin, and the coating parameter is fluidized coating drying. 2ml / min. After spraying, continue to dry for 20 minutes, granulate, take pellets with a diameter of 80-800 μm, clarithromycin content: 48.5%, and other items meet the quality standards.
[0040] (2) Preparation of clarithromycin dry suspension
[0041] Specifications: Each bag contains clarithromycin 100mg, 2g / bag, when taking i...
Embodiment 2
[0045] Preparation of clarithromycin debitter granules
[0046] (1) Preparation of coating solution:
[0047] Solution A: Take 300g of glyceryl monostearate, 12g of Sipan 20-100, 500g of pure water, and stir well;
[0048] Solution B: Take 5880g of Eudragit L30D55, 120g of butylene sebacate, 180g of diethyl phthalate, 100g of microcrystalline cellulose, 5100g of pure water, and stir well.
[0049] Solution C: Take 100g of No. II acrylic resin, 1000g of 95% ethanol, fully stir to dissolve,
[0050] Then, add pure water to solution A\solution B and solution C to 19250g, set aside.
[0051] (2) Coating
[0052] Take 2000g 60 mesh clarithromycin, the coating parameters are fluidized coating and drying, first preheat the coating system to 38°C, and spray coating, control the inlet temperature to 45°C, the atomization pressure to 0.32MPa, and the spray liquid flow 60ml / min. After spraying, continue to dry for 20 minutes, granulate, take pellets with a diameter of 80-800 μm, clari...
Embodiment 3
[0054] Preparation of clarithromycin debitter granules
[0055] (1) Preparation of coating solution:
[0056] Solution A: Take polyethylene glycol 200-20000 450g, Tween-8015g, pure water 1800g, stir well;
[0057] Solution B: Take Eudragit L30D55 5880g, butylene sebacate 300g, microcrystalline cellulose 100g, pure water 3500g, stir well.
[0058] Solution C: Take 120g of No. III acrylic resin, 2280g of 95% ethanol, fully stir to dissolve,
[0059] Then, add pure water to solution A\solution B and solution C to 21300g, set aside.
[0060] (2) Coating
[0061] Take 2000g 120 mesh clarithromycin, the coating parameters are fluidized coating and drying, first preheat the coating system to 38°C, and carry out spray coating, control the inlet temperature at 60°C, the atomization pressure at 0.45MPa, and the spray liquid flow rate 120ml / min. After spraying, continue to dry for 20 minutes, granulate, take pellets with a diameter of 80-800 μm, clarithromycin content: 30.5%, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com