Enteric coated preparation of Clarithromycin
A technology of enteric-coated preparations and clarithromycin, which is applied in the field of clarithromycin enteric-coated preparations and its preparation, can solve the problems of low bioavailability, achieve the effects of reducing damage, improving bioavailability, and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Enteric-coated tablets using clarithromycin as raw material
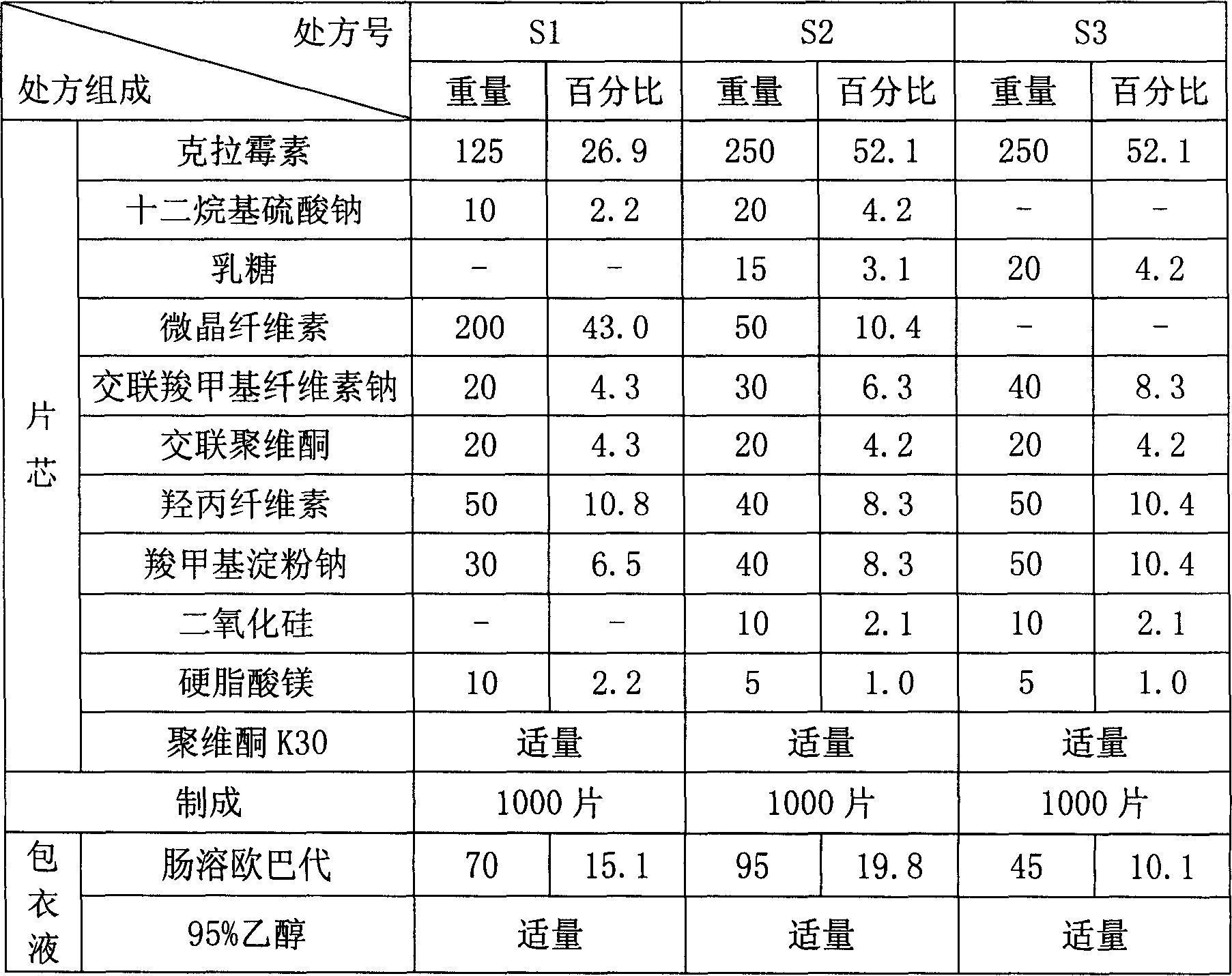
[0041]
[0042] Weigh clarithromycin, lactose, microcrystalline cellulose, 1 / 2 amount of crospovidone, croscarmellose sodium, hydroxypropyl cellulose, 1 / 2 amount through a 80-mesh sieve according to the prescription amount sodium carboxymethyl starch, after mixing evenly, add an appropriate amount of 10% povidone aqueous solution, granulate, and dry the dry granules with 1 / 2 amount of crospovidone and 1 / 2 amount of sodium carboxymethyl starch, Silicon dioxide and magnesium stearate are mixed evenly, the content is measured, and the determined amount is pressed into tablets with a tablet machine.
[0043] The prepared tablet cores are weighed and placed in a coating pan, and coated with a coating solution to obtain enteric-coated tablets.
Embodiment 2
[0044] Example 2: Enteric-coated tablets using clarithromycin as raw material
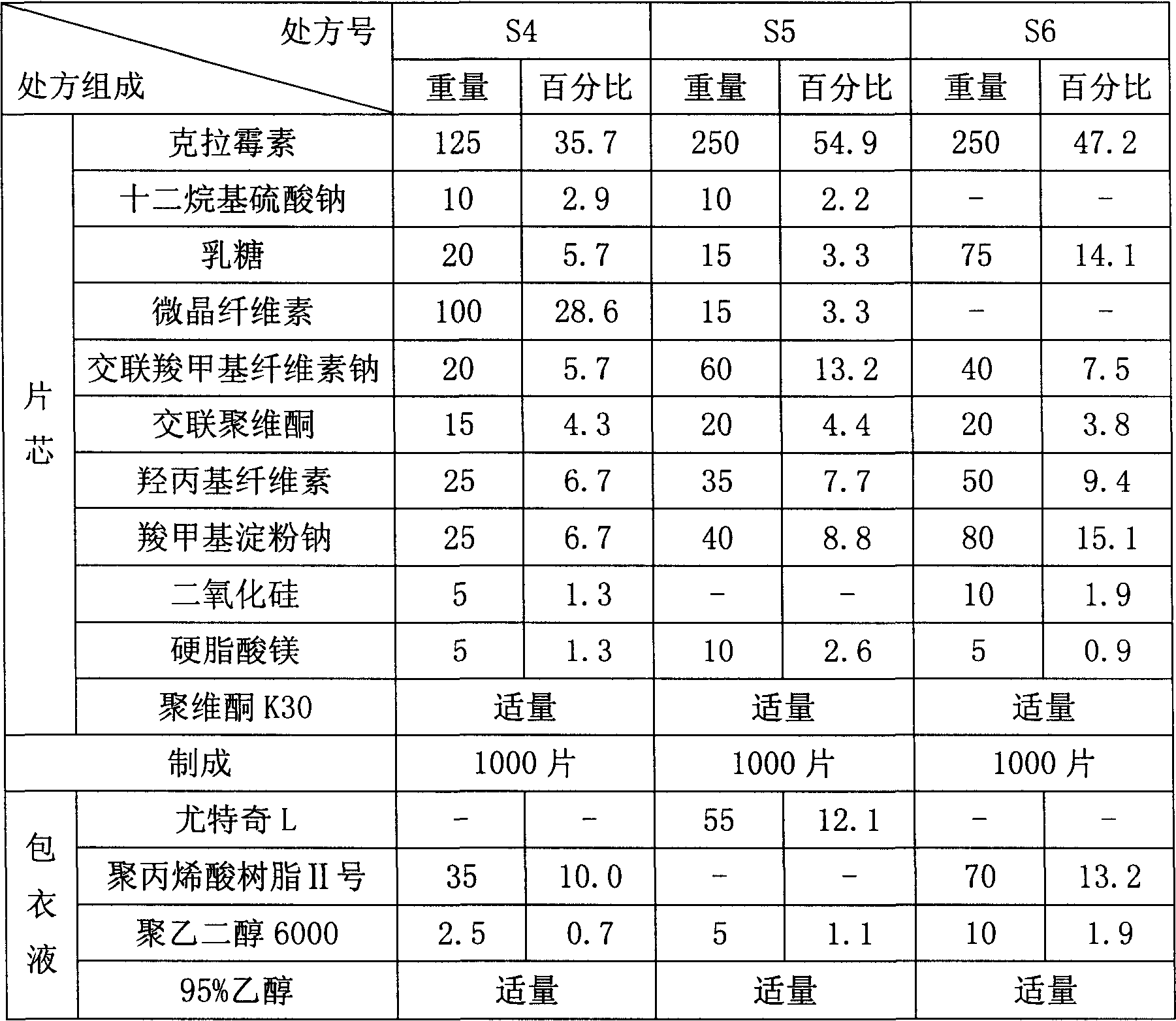
[0045]
[0046] Concrete preparation method is with embodiment 1.
Embodiment 3
[0047] Example 3: Enteric-coated tablets using clarithromycin salt as raw material
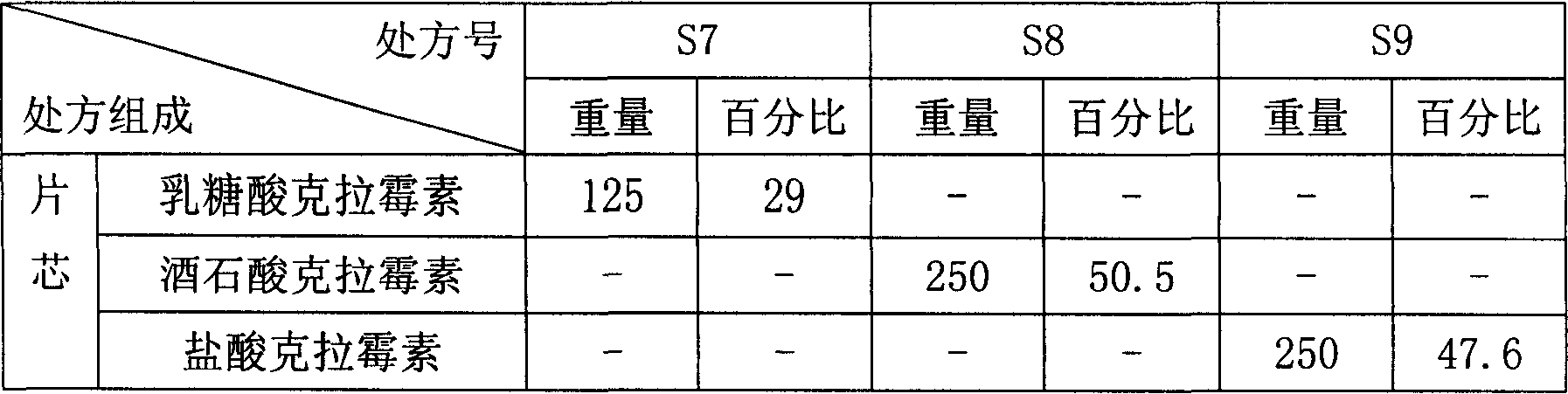
[0048]
[0049] Sodium dodecyl sulfate
[0050] Concrete preparation method is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com