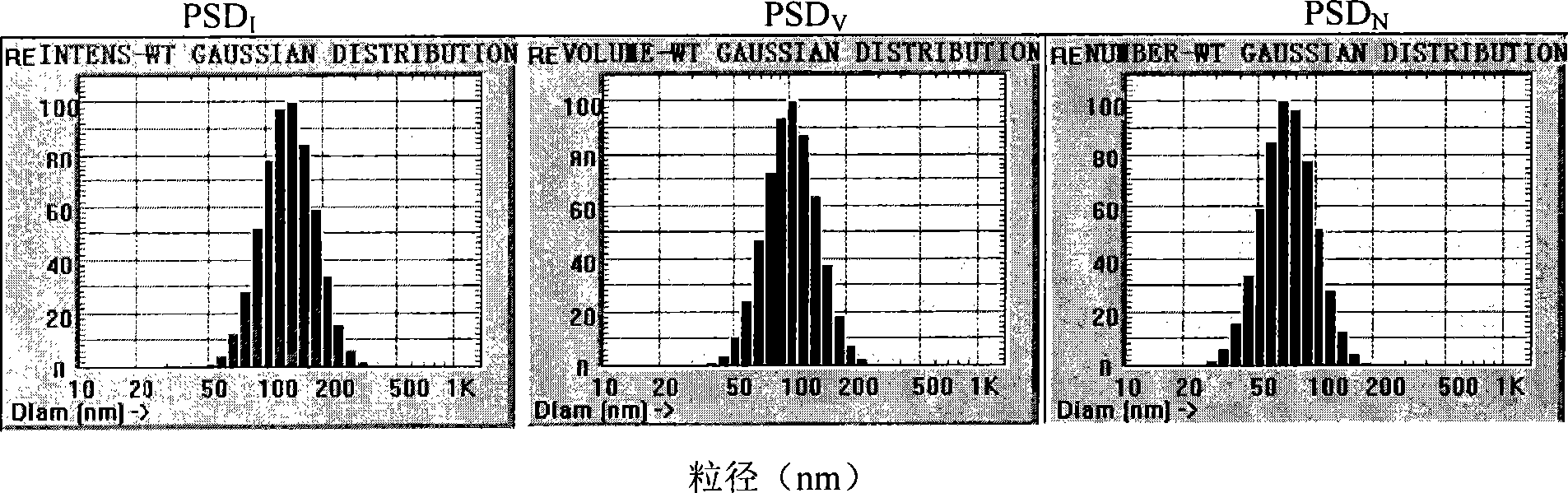
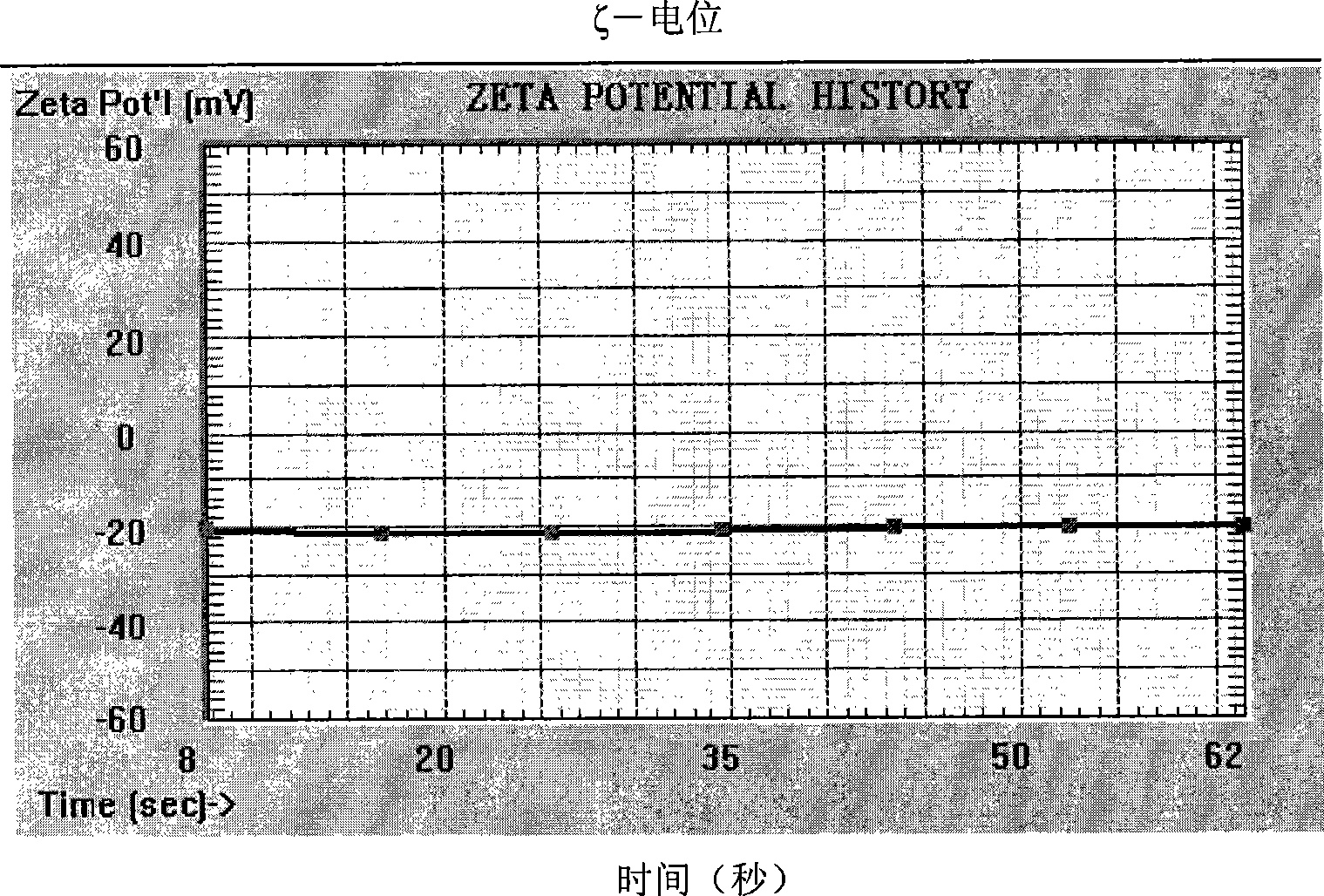
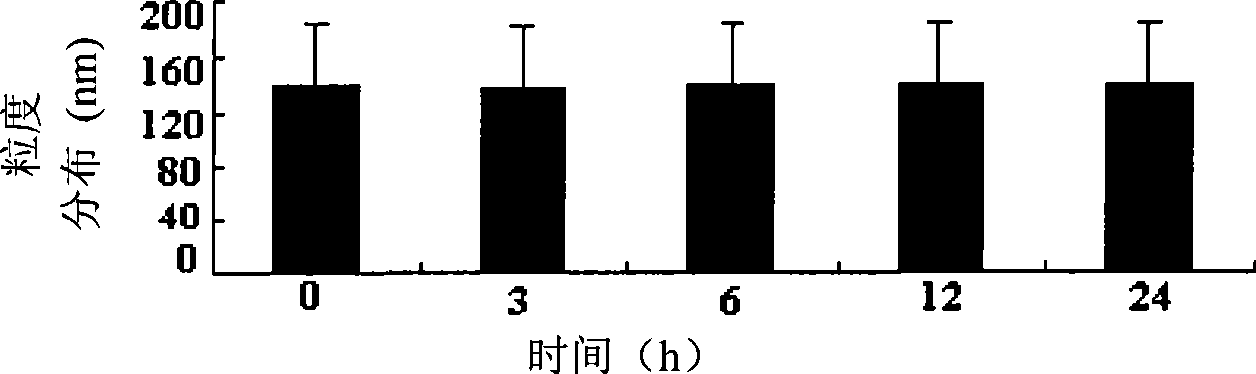
Clarithromycin sub-microemulsion injection and preparation method thereof
A technology for clarithromycin and injection, applied in the field of clarithromycin submicroemulsion injection and preparation thereof, can solve the problems of not mentioning sterilization, limiting sterilization methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Formula 1 Clarithromycin content 250mg / 100mL
[0071] Based on 100mL injection
[0072] Clarithromycin phospholipid complex 0.25g as clarithromycin
[0073] Medium chain fatty acid triglycerides (MCT) 16g
[0074] Soybean Oil for Injection (LCT) 4g
[0075] Soy Lecithin 1g
[0076] Pluronic F-68 0.2g
[0077] Tween-80 0.2g
[0078] L-cysteine 0.02g
[0079] Sodium oleate 0.1g
[0080] Glycerin 2.5g
[0081] The rest is water for injection
[0082] Among them, the mass ratio of clarithromycin to soybean lecithin in the clarithromycin phospholipid complex is 1:10.
[0083] Preparation of clarithromycin phospholipid complex: place 0.25 g of clarithromycin and 2.5 g of soybean lecithin in a round bottom flask, and slowly add absolute ethanol under stirring until the clarithromycin is completely integrated into the soybean lecithin, and continue adding After anhydrous ethanol is 1 / 3 of the added amount, stop adding anhydrous ethanol and react in anhydrous ethanol at ...
Embodiment 2
[0090] Example 2 Formula 2 Claramycin content 100mg / 100mL
[0091] Based on 100mL injection
[0092] Clarithromycin phospholipid complex 0.1g as clarithromycin
[0093]Medium chain fatty acid triglycerides (MCT) 12g
[0094] Soybean Oil for Injection (LCT) 8g
[0095] Soy Lecithin 0.5g
[0096] Pluronic F-68 0.4g
[0097] Tween-80 0.2g
[0098] L-cysteine 0.02g
[0099] Sodium oleate 0.1g
[0100] Glycerin 2.5g
[0101] The rest is water for injection
[0102] Among them, the mass ratio of clarithromycin to soybean lecithin in the clarithromycin phospholipid complex is 1:12.
[0103] Preparation of clarithromycin phospholipid complex: 0.1 g of clarithromycin and 1.2 g of soybean lecithin were used to prepare the complex in the same manner as in Example 1.
[0104] Preparation of clarithromycin submicron emulsion injection:
[0105] Step 1: Disperse 2.5g of glycerol for injection, 0.4g of Pluronic F-68, 0.2g of Tween-80, 0.1g of sodium oleate for injection, and 0.02g of L-cystein...
Embodiment 3
[0110] Example 3 Formula 3 Claramycin content 500mg / 100mL
[0111] Based on 100mL injection
[0112] Clarithromycin phospholipid complex 0.5g as clarithromycin
[0113] Medium chain fatty acid triglycerides (MCT) 18g
[0114] Soybean Oil for Injection (LCT) 2g
[0115] Soy Lecithin 0.2g
[0116] Pluronic F-68 0.4g
[0117] Tween-80 0.3g
[0118] L-cysteine 0.02g
[0119] Sodium oleate 0.1g
[0120] Glycerin 2.5g
[0121] The rest is water for injection
[0122] Among them, the mass ratio of clarithromycin to soybean lecithin in the clarithromycin phospholipid complex is 1:8.
[0123] The clarithromycin phospholipid complex was prepared in the same manner as in Example 1.
[0124] Preparation of clarithromycin submicron emulsion injection:
[0125] Step 1: Disperse 2.5g of glycerol for injection, 0.4g of Pluronic F-68, 0.3g of Tween-80, 0.1g of sodium oleate for injection, and 0.02g of L-cysteine in an appropriate amount of water for injection, and stir it magnetically Heat to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com