Camptothecin-adriamycin prodrug and preparation method and application thereof
A camptothecin and doxorubicin technology, which is applied in the field of camptothecin-doxorubicin prodrugs, can solve the problems of low drug loading, complex synthesis, and not the best curative effect of high molecular polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
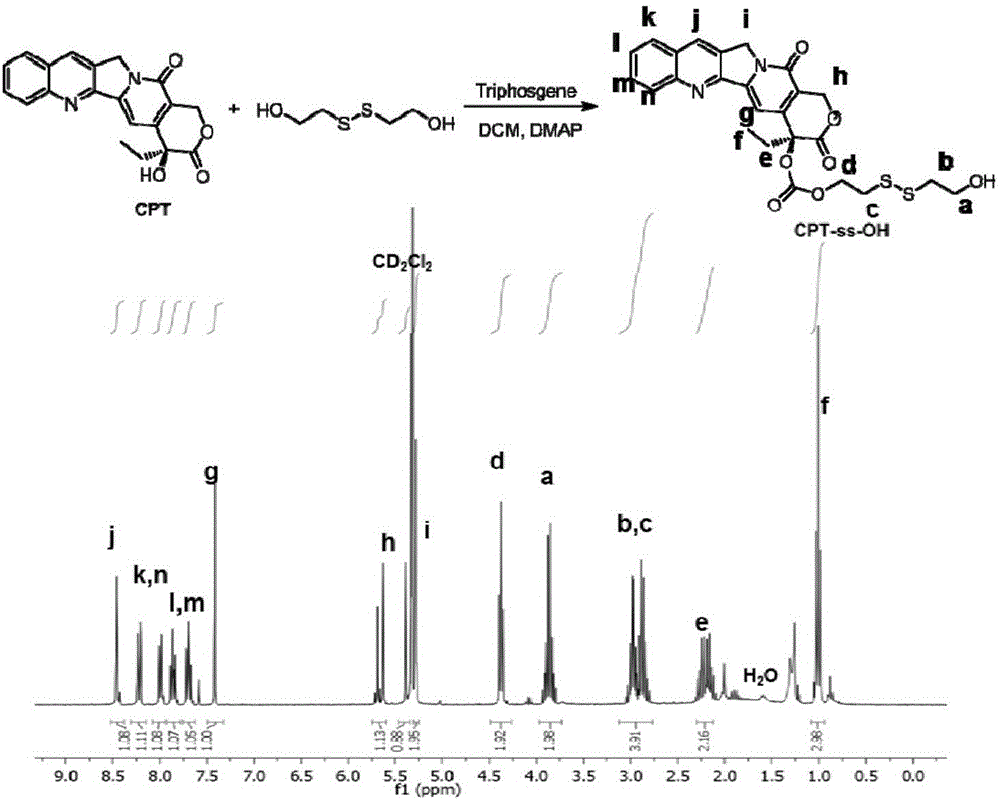
[0070] Embodiment 1: the preparation of CPT-ss-OH
[0071] Under stirring, a solution of 4-dimethylaminopyridine (1.05 g, 8.60 mmol) in 10 mL of dichloromethane was added dropwise to a solution of camptothecin (1.0 g, 2.87 mmol) and triphosgene (0.315 g, 1.06 mmol). The mixture was suspended in anhydrous dichloromethane (200 mL). After stirring for 30 minutes, 2,2'-dithiodiethanol (8.60 g, 55.8 mmol) in anhydrous tetrahydrofuran (25 mL) was added and the reaction mixture was stirred at room temperature overnight. The mixture was washed with 50 mM aqueous hydrochloric acid (2×100 mL), water (1×100 mL) and saturated brine (1×100 mL). The organic layer was separated and dried over anhydrous sodium sulfate. The solution was concentrated by rotary evaporator and purified by flash chromatography using a prepacked silica column. Yield: 1.05 g (69% yield). 1 H NMR (300MHz, CD 2 Cl 2 )δ8.46(s, 1H), 8.22(d, J=8.3Hz, 1H), 7.99(dd, J=8.2, 1.1Hz, 1H), 7.86(ddd, J=6.9, 6.5Hz, 1H), 7....
Embodiment 2
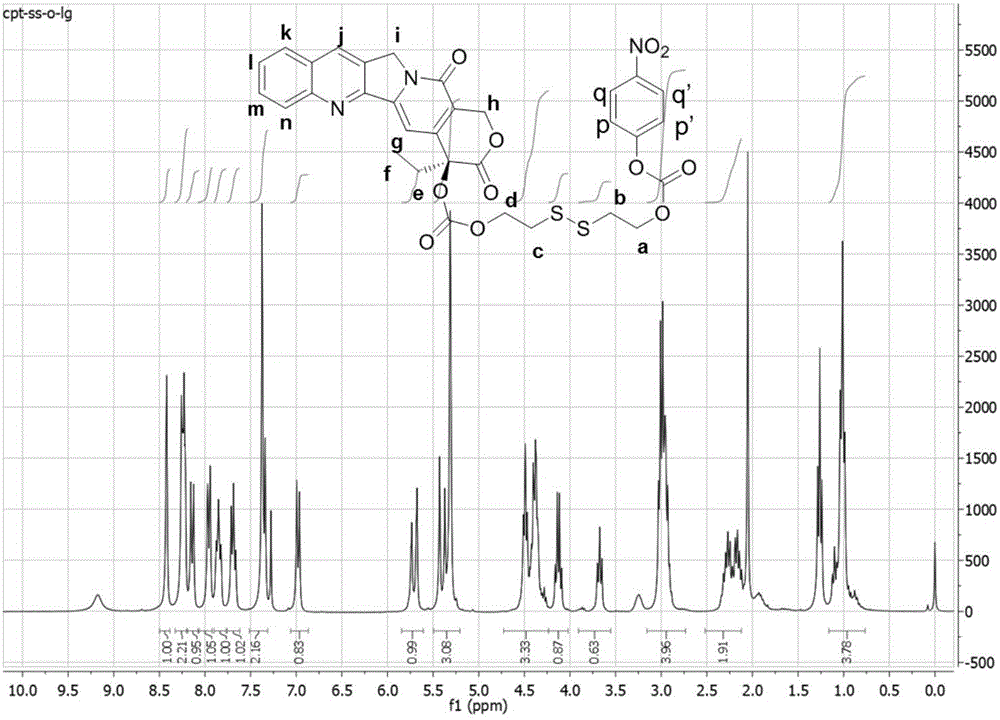
[0072] Embodiment 2: Preparation of CPT-ss-LG
[0073] Under stirring, 4-nitrophenyl chloroformate (372mg, 1.85mmol), CPT-ss-OH (100mg, 0.184mmol) prepared in Example 1 and triethylamine (TEA, 224mg, 2.22mmol) were dissolved in water DCM, the reaction mixture was stirred at room temperature overnight, then purified by flash chromatography (Teledyne ISCO CombiFlash) using a prepacked silica column. A gradient mixture of ethyl acetate and hexane was used as eluent. Yield: 102 mg (80% yield). 1 H NMR (300MHz, CDCl 3 )δ8.40(s, 1H), 8.27-8.16(m, 3H), 8.12(d, J=9.0Hz, 1H), 7.94(d, J=8.0Hz, (t, J=7.3Hz, 1H) , 7.67(t, J=7.5Hz, 1H), 7.34(d, J=10.2Hz, J=17.2Hz, 1H), 5.43-5.32(m, 1H), 5.29(s, 2H), 4.55-4.41( m, 2H), 4.42-4.31 2H, 1H), 2.95 (td, J = 13.9, 6.3 Hz, 4H), 2.35-2.09 (m, 2H), 1.00 (t, J = 7.3 Hz, 3H). figure 2 for CPT-ss-LG 1 The H NMR spectrum proves that the compound was successfully prepared.
Embodiment 3
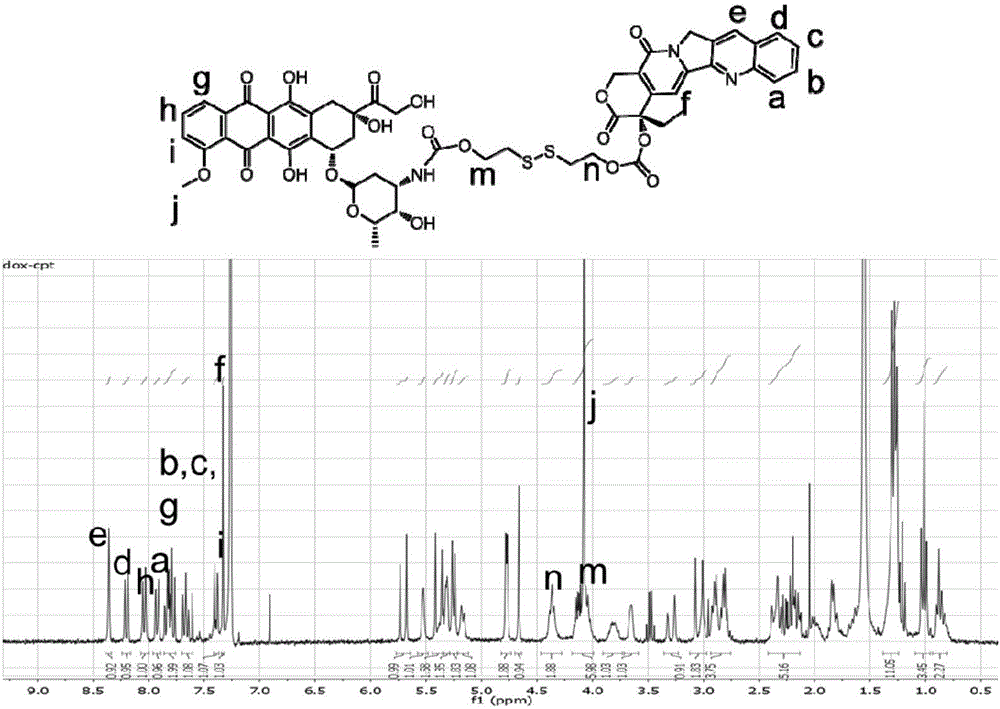
[0074] Embodiment 3: Preparation of CPT-ss-DOX
[0075] CPT-ss-LG (43 mg, 0.062 mmol), DOX.HCl (38 mg, 0.066 mmol), TEA (63 mg, 0.62 mmol) prepared in Example 2 were mixed in 3 mL of DMF and stirred under nitrogen for 1 day. The reaction was quenched by the addition of excess acetic acid and purified by preparative HPLC using acetonitrile and 0.1% trifluoroacetic acid in water (gradient: 20-95% acetonitrile). The collected purified product was lyophilized and stored at -20°C for later use. Yield: 35 mg (51% yield). 1 H NMR (300MHz, CDCl 3)δ8.36(s, 1H), 8.20(d, J=8.3Hz, 1H), 8.04(d, J=7.0Hz, 1H), 7.92(d, J=7.7, 1H), 7.86-7.73(m , 3H), 7.71-7.58(m, 2H), 7.43-7.36(m, 1H), 7.33(s, 1H), 5.70(d, J=17.2Hz, 2H) 2H), 5.45-5.14(m, 7H ), 4.77(d, J=4.3Hz, 2H), 4.66(s, 1H), 4.37(s, 2H), 4.18-4.01(s, 2H), 3.65(s, 2H), 3.32(s, 1H) , 3.26(s, 1H), 3.07(s, 1H), 3.01(s, 1H), 2.89(d, J=2H), 2.81(dd, J=10.7, 6.2Hz, 2H), 2.43-2.09(m , 7H), 2.02-1.75 (m, 5H), 1.36-1.25 (m, 10H), 1.02 (t, 7.5Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com