Method for analyzing N-nitrosodimethylamine and N-nitrosodiethylamine in argatroban bulk drug or preparation
A technology of nitrosodimethylamine and nitrosodiethylamine, which is applied in the field of drug analysis, can solve problems such as no reports, and achieve the effects of simple operation, good stability and accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] Preparation of test product (argatroban bulk drug) solution 1: Accurately weigh about 100 mg of argatroban bulk drug 1 and place in a 10ml measuring bottle, add methanol to dissolve and dilute to the scale, shake well, and obtain a concentration of 10 mg / ml The test solution 1.
[0062] Preparation of the test product (argatroban injection) solution 2: take argatroban injection (10mg / 2ml) and inject directly.
[0063] The prescription of argatroban injection is: 2ml injection contains argatroban raw material drug 1 10mg, ethanol 300mg, glycerin 900mg, and the rest is water for injection.
Embodiment 1
[0064] Example 1: System Suitability
[0065] (1) Chromatographic conditions:
[0066] Detection instrument: Waters XEVO TQ-S ultra-high performance liquid mass spectrometer
[0067] Ultra-high performance liquid chromatography conditions:
[0068] Chromatographic column: phenyl chromatographic column: ACE 5phenyl 4.6×100mm, 5μm (particle size);
[0069] Flow rate: 0.8ml / min; Column temperature: 30°C; Injection volume: 10μl;
[0070] Mobile phase A: Precisely measure 0.5g of formic acid, dissolve in 500ml of ultrapure water, and degas it by ultrasonic;
[0071] Mobile phase B: methanol, ultrasonic degassing;
[0072] Gradient elution table:
[0073] time (min) Mobile phase A(%) Mobile phase B(%) 0 90 10 6 10 90 12 10 90 12.1 90 10 15 90 10
[0074] The mass spectrometry conditions are as follows:
[0075] Mass Spectrometer: Triple Quadrupole Mass Spectrometer; Mass Spectrometry Method Mode: Multiple Reaction Monitoring Scan...
Embodiment 2
[0089] Example 2: Specificity
[0090] (1) Test method:
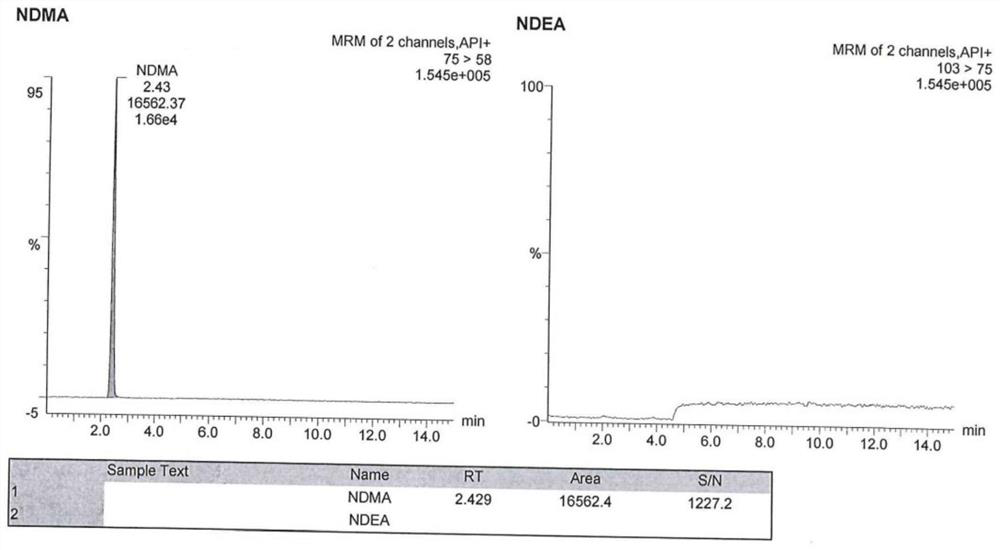
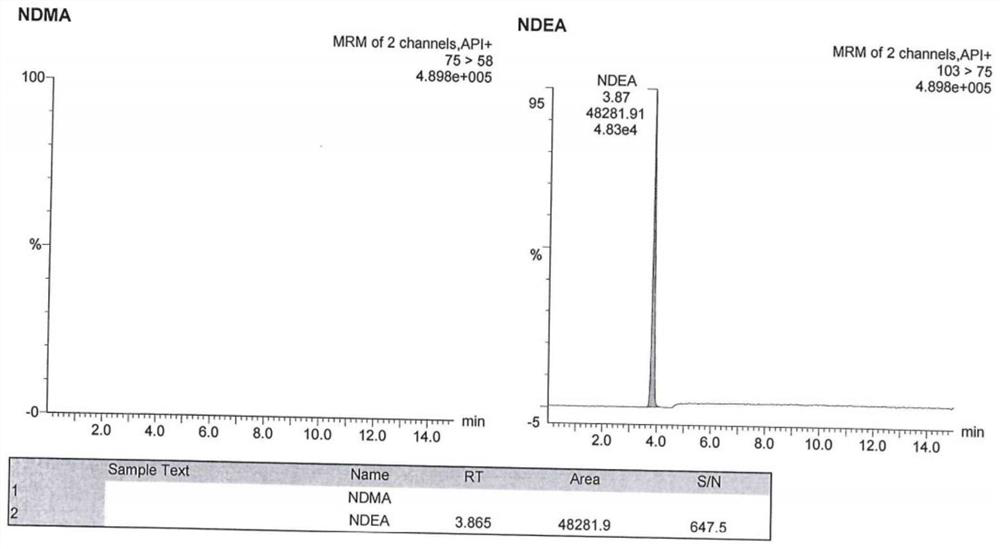
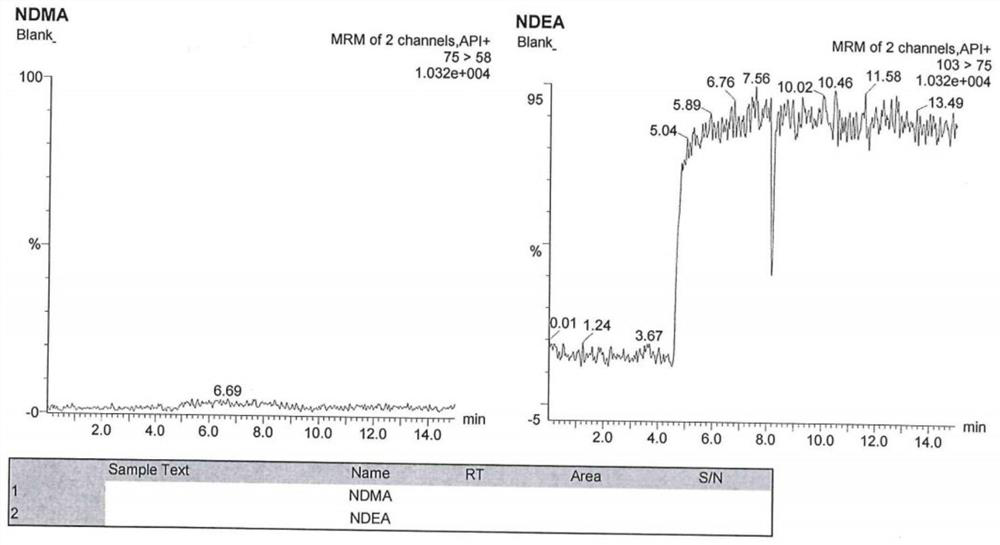
[0091] The chromatographic conditions are the same as those in Example 1, and an appropriate amount of N-nitrosodimethylamine reference substance stock solution and N-nitrosodiethylamine reference substance stock solution are taken respectively, and diluted with methanol respectively to obtain N-nitrosodimethylamine Amine-specific solution, N-nitrosodiethylamine-specific solution.
[0092] Inject blank solution (methanol), blank excipients (mixed solution of ethanol and glycerin), N-nitrosodimethylamine specific solution, N-nitrosodiethylamine specific solution, and argatroban reference substance solution.
[0093] Wherein the detection method of argatroban reference substance solution is high performance liquid chromatography, chromatographic column, flow rate, column temperature, injection volume, mobile phase A, mobile phase B, gradient elution method are the same as before; detection wavelength 226nm.
[0094] (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com