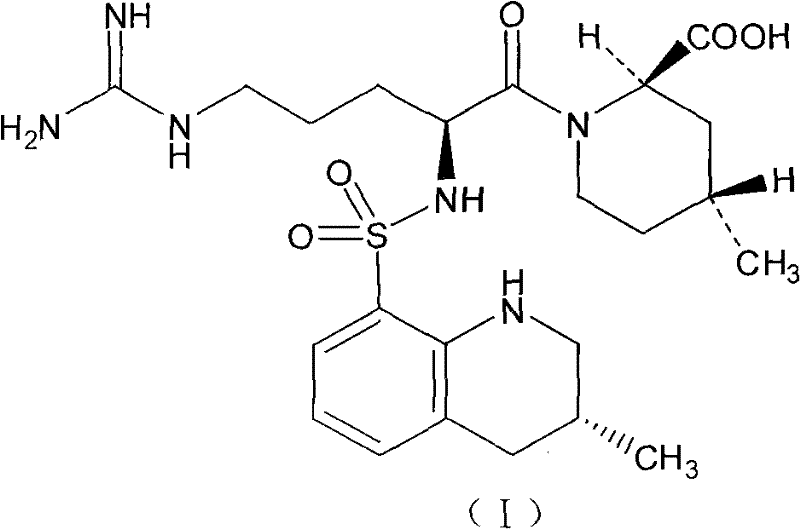
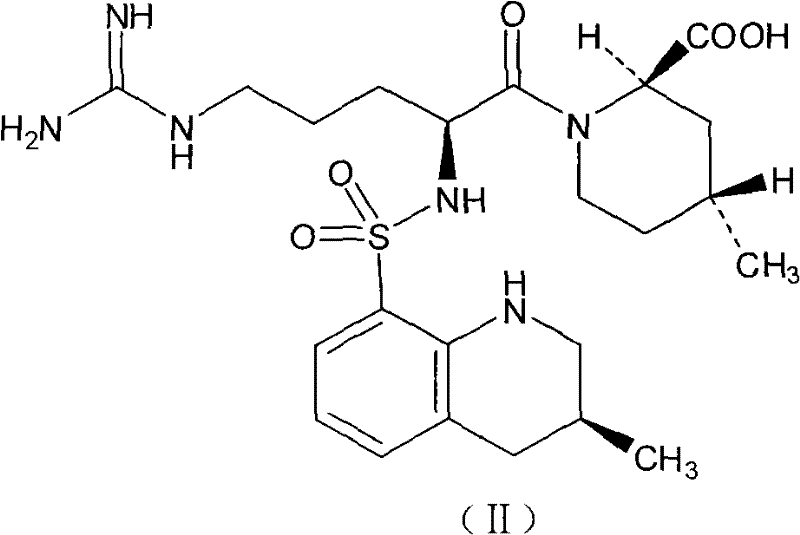
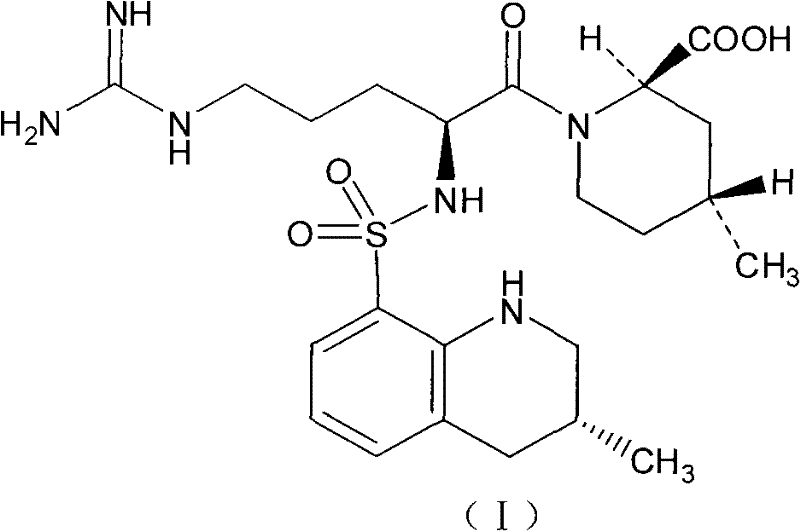
Injection preparation containing argatroban
A technology for argatroban and injection, which is applied in the field of medicine, can solve the problems of high disability rate and achieve the effect of stable quality and low pharmacological toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Argatroban sterile powder injection
[0041] prescription:
[0042]
[0043] Preparation:
[0044] 1. Take 400g of absolute ethanol, add D-sorbitol and stir to dissolve to form a solution.
[0045] 2. Take the aseptic powder of argatroban, quantitatively fill it into 3ml vials, stopper 0.25g per bottle, and crimp the cap.
[0046] 3. When in use, use the solution obtained in step 1 to dissolve the sterile powder obtained in step 2, and dilute it with 0.9% NaCl injection, or 5% glucose injection, or Lactated Ringer's injection to a concentration of 1 mg / ml. The dilution is Argatroban may crystallize out, but repeated shaking can dissolve it.
Embodiment 2
[0047] Embodiment 2: Argatroban small volume injection
[0048] prescription:
[0049]
[0050]
[0051] Preparation:
[0052] Take 400g of absolute ethanol, add D-sorbitol and stir to dissolve, add argatroban, stir to dissolve, add 0.1% activated carbon for needles according to the prepared amount, heat the liquid to about 60°C, stir for 20min, filter and decarbonize, Add water for injection to the total amount, and detect intermediates. After passing the test of the intermediate, fine filter with a 0.22 μm microporous membrane, fill in vials, 2.5 ml per vial, stopper, and cap. Moist heat sterilization, you can.
Embodiment 3
[0053] Embodiment 3: Argatroban small volume injection
[0054] prescription:
[0055]
[0056] Preparation:
[0057] Take water for injection accounting for about 80% of the total volume, add sodium chloride, stir to dissolve, add argatroban, stir to dissolve, add 0.1% activated carbon for needles according to the prepared amount, heat the liquid medicine to about 60°C, stir for 20 minutes, After filtering and decarbonizing, add physiological saline to the total amount, and detect intermediates. After passing the test of the intermediate, fine filter with a 0.22 μm microporous membrane, fill in vials, 2.5 ml per vial, stopper, and cap. Moist heat sterilization, you can.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com