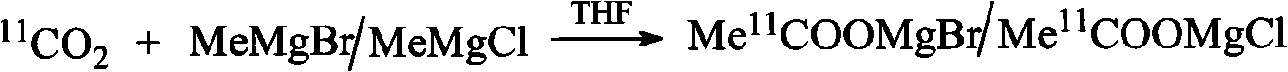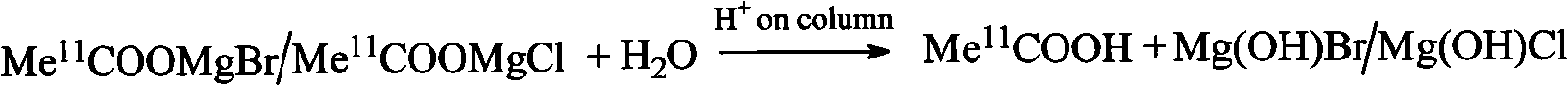Patents
Literature
43 results about "Parenteral solutions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Parenteral solutions are packaged as large volume parenteral (LVP) solutions and small volume parenteral (SVP) solutions. LVP solutions are typically bags or bottles containing larger volumes of intravenous solutions.
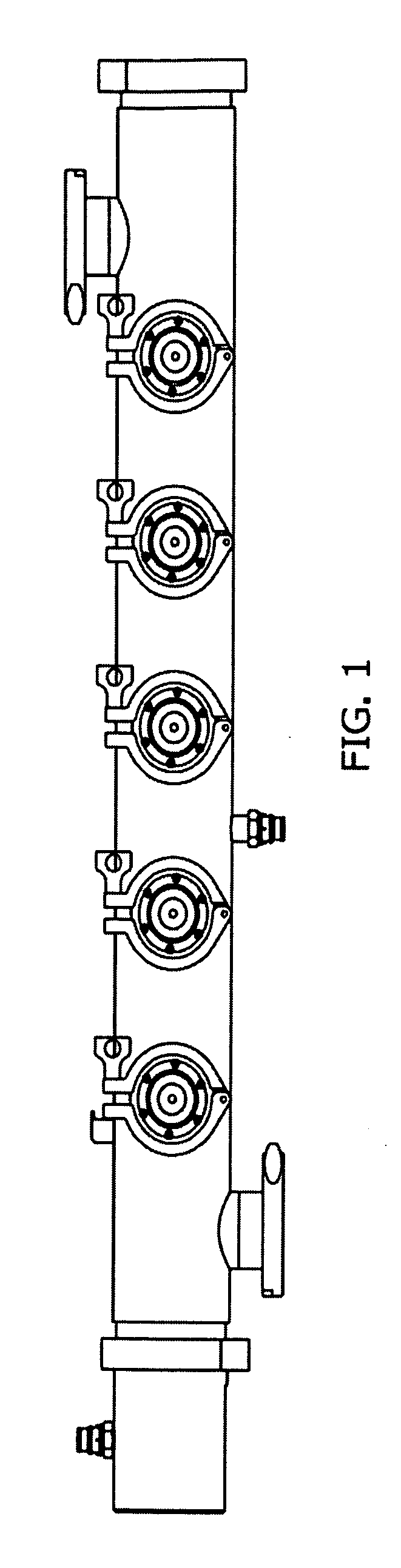
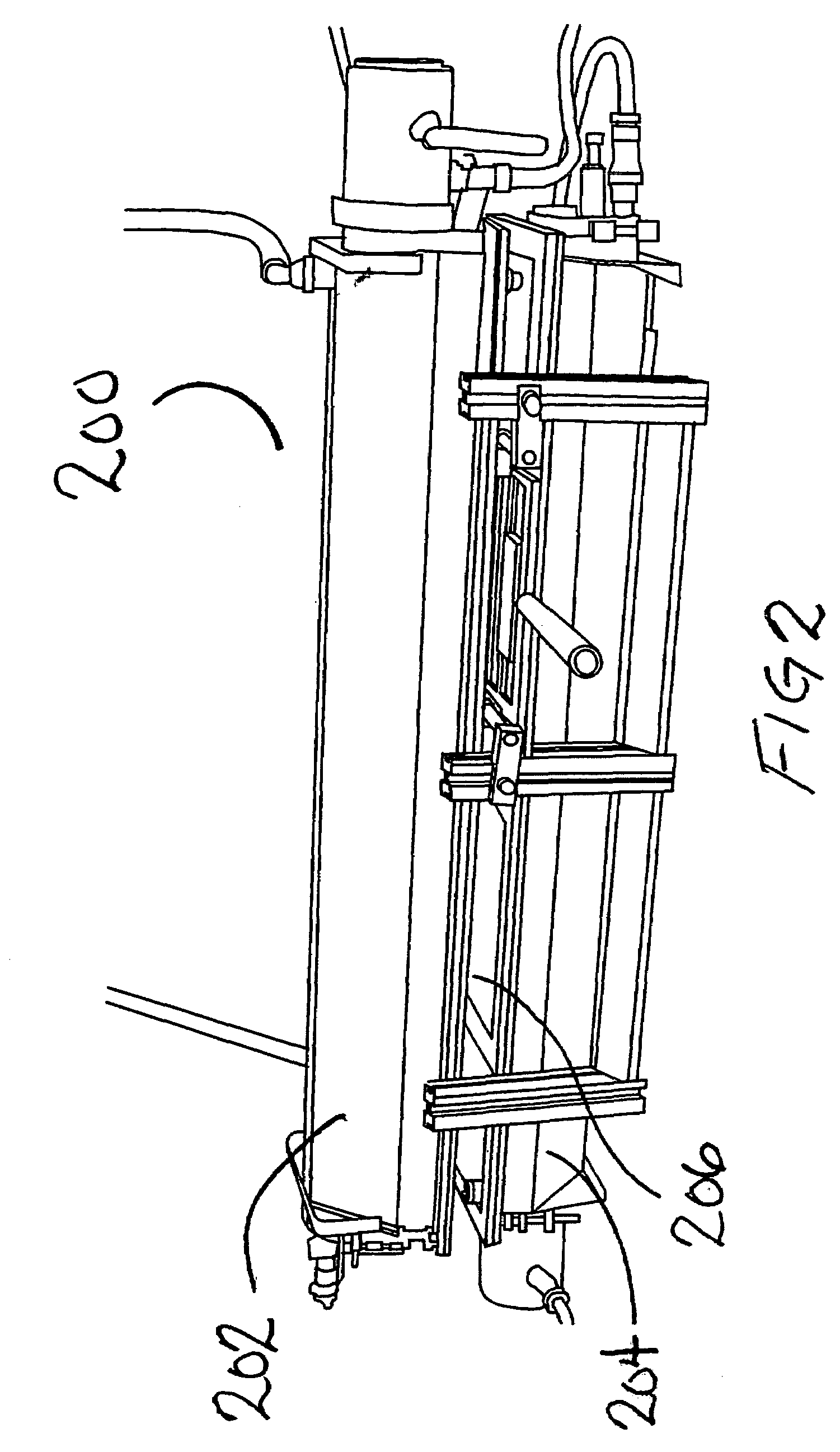
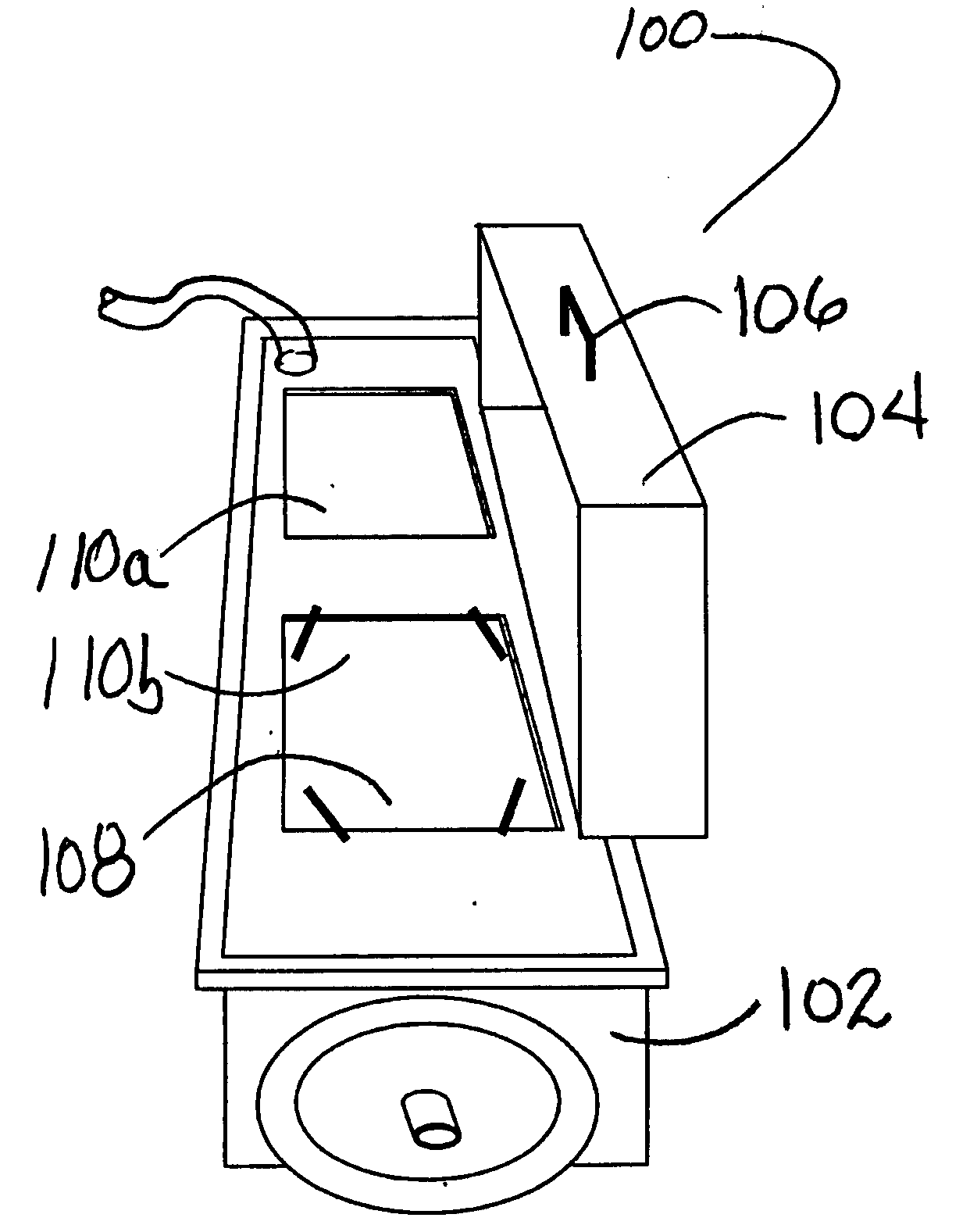
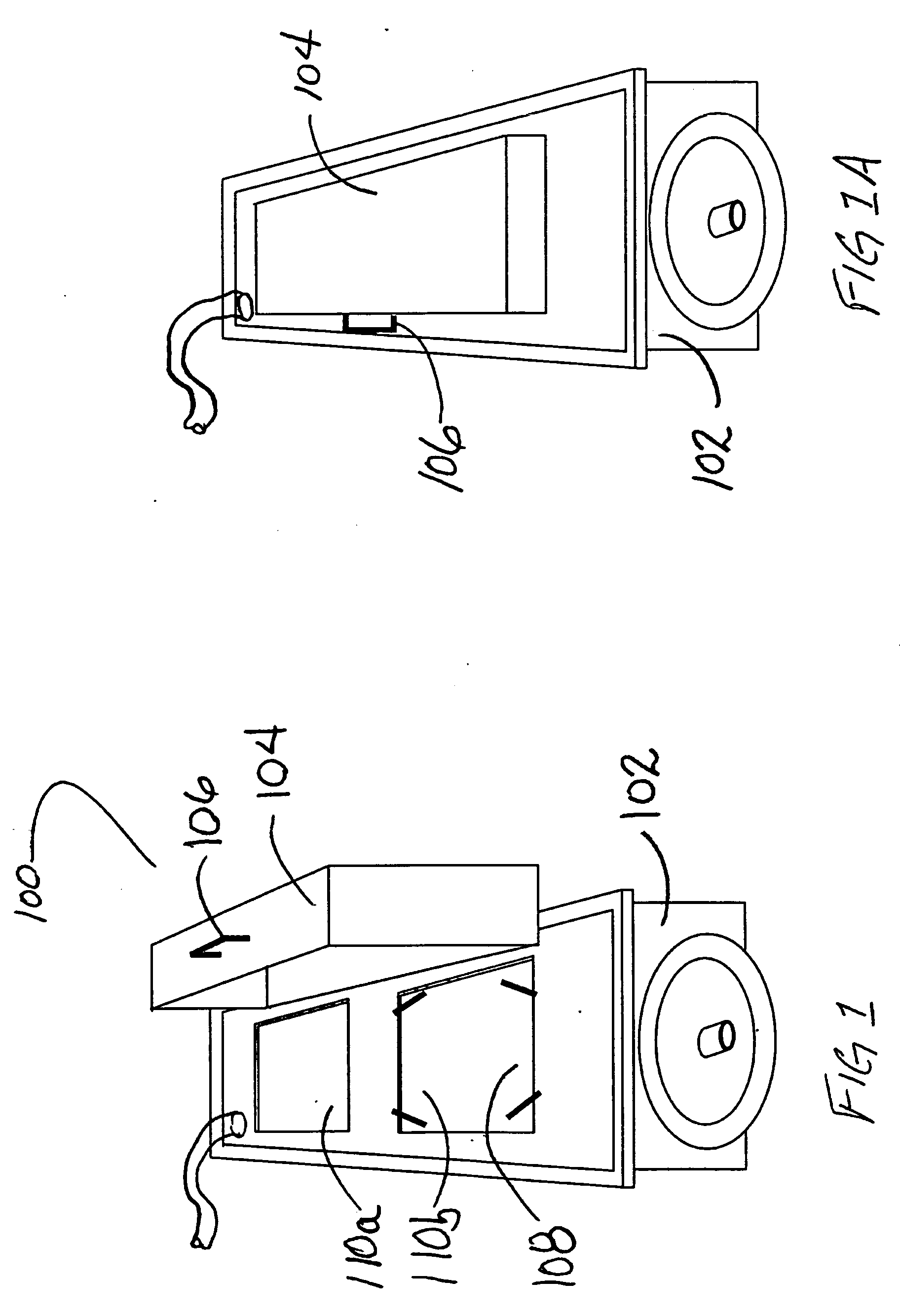
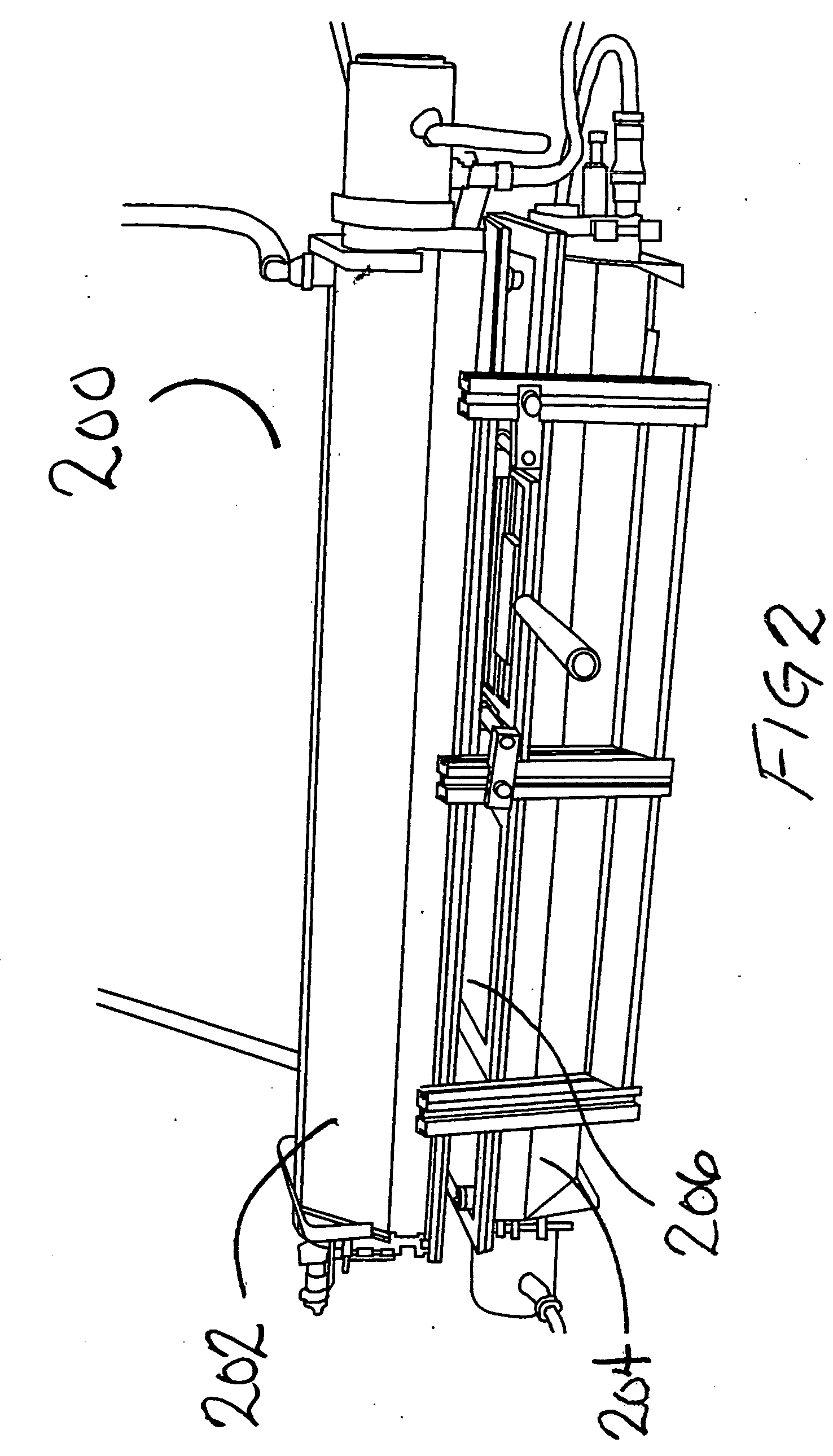
System and method for container sterilization using UV light source
InactiveUS20090274576A1Wide applicabilityImprove the bactericidal effectPackage sterilisationFood preservationHigh intensityPack material
Packaging materials and containers may be treated with sterilization dosages internally and externally using monochromatic, continuous wave, high-intensity, incoherent light in single and / or multiple light source configurations. The treatment systems and methods preserve physical and performance properties of the packaging / container while achieving a desired level of sterilization. The sterilized materials may be adapted for extended shelf life (ESL) products. The disclosed treatment systems and methods may also be used for sterilization of food and beverage products (either prepackaged or post packaged), medicines, pharmaceuticals, vitamins, infusion products, clinical and / or non-clinical solutions and systems, enteral and / or parenteral solutions and systems, and the like.
Owner:TRITON THALASSIC TECH
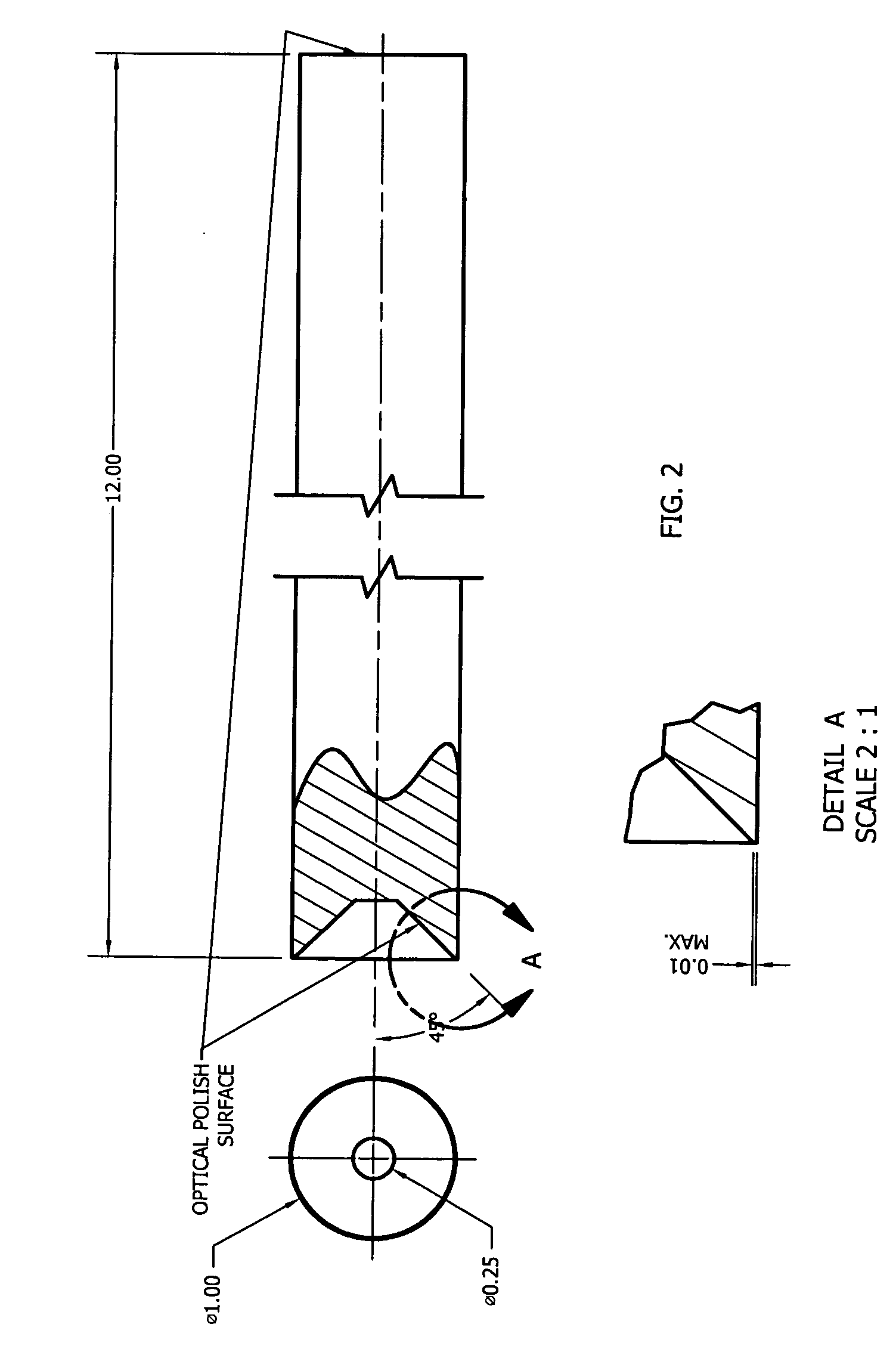
System and method for product sterilization using UV light source
InactiveUS7217936B2Efficient inactivationEffective treatmentRadiation pyrometryMaterial analysis using wave/particle radiationHigh intensityParenteral solutions
Owner:TRITON THALASSIC TECH
System and method for product sterilization using UV light source
InactiveUS20050173652A1Efficient inactivationEffective treatmentMaterial analysis using wave/particle radiationElectric discharge tubesMonochromatic colorHigh intensity
System and method for sterilization, using UV light source(s), of products, e.g., polymer-based products, whether positioned within or external to their packaging, using monochromatic, continuous wave, high-intensity, incoherent light in single and / or multiple light source configurations. The treatment system(s) and method(s) may be used for sterilization of alternative products, including, for example, food products such as meat and poultry, enteral and / or parenteral solutions and systems, and the like.
Owner:TRITON THALASSIC TECH
Coenzyme Q10 injection
InactiveCN101278907AStrong solubilizing abilityLow toxicityOrganic active ingredientsPharmaceutical delivery mechanismParenteral nutritionHydroxystearic Acid
The invention discloses the novel combined coenzyme Q10 parenteral solution, mainly containing the coenzyme Q10 as active component, polyethylene glycol 15-hydroxyl stearate as solubilizing agent and water for injection as solvent. One or more solvents of cosolvent, acidity regulator, osmoregulator and stabilizing agents can be also added. Low hemolyzation, extreme low histamine release and higher physiological tolerance of a novel solubilizing agent Solutol HS 15 adopted by the parenteral solution remarkably improves clinical medication security and the compliance of a patient; and the parenteral solution has the advantages of good stability, longer period of validity, higher medication convenience for a clinician, better storage and stable transportation, higher security for the clinical medication and better compliance of the patient. The parenteral solution also has the advantages of simple preparation technology, simple and convenient quality control and lower production cost, thereby being beneficial to industrialization production.
Owner:郑微
Limpid parenteral solution of 2,6-diisoprophylphenol as an anaesthetic drug and 2.5-di-O-methyl-1.4;3.6-dianhydro-D-glucitol as a solvent for making clear I. V. formulation
This invention relates to a novel pharmaceutical composition which may be administered parenterally to a mammal for the production of general anaesthesia, specifically to a limpid, stable, injectable pharmaceutical formulation of 2,6-diisopropylphenol. The formulation comprises 2,6-diisopropylphenol, a compound of the following general formula (I) wherein R1 and R2 can be the same or different and are hydrogen or lower alkyl group containing 1-3 carbons or acetate group and optionally water and / or one or more antioxidants.
Owner:THEMIS CHEM LTD
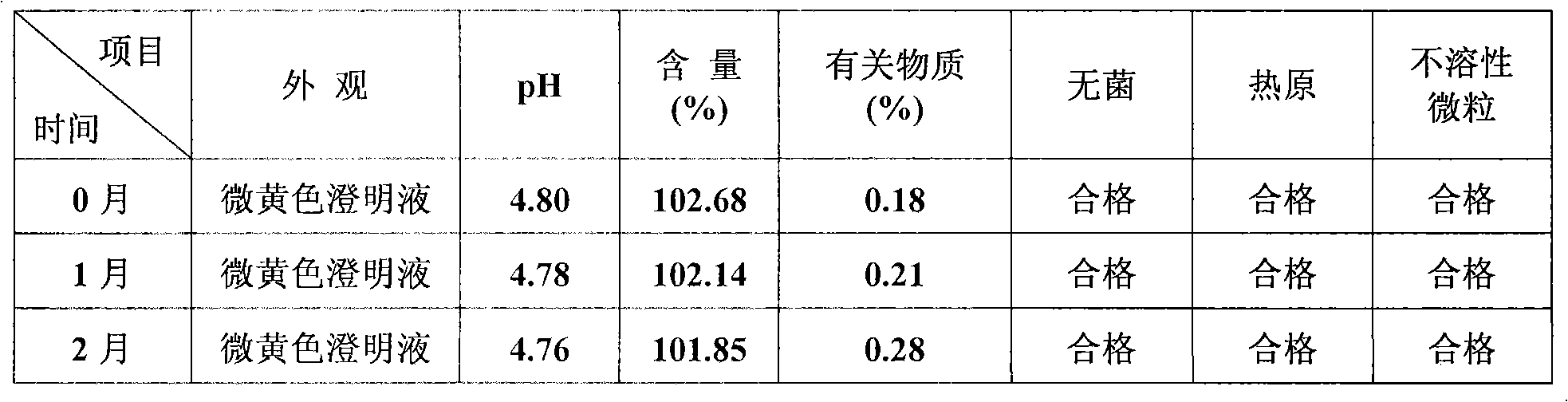
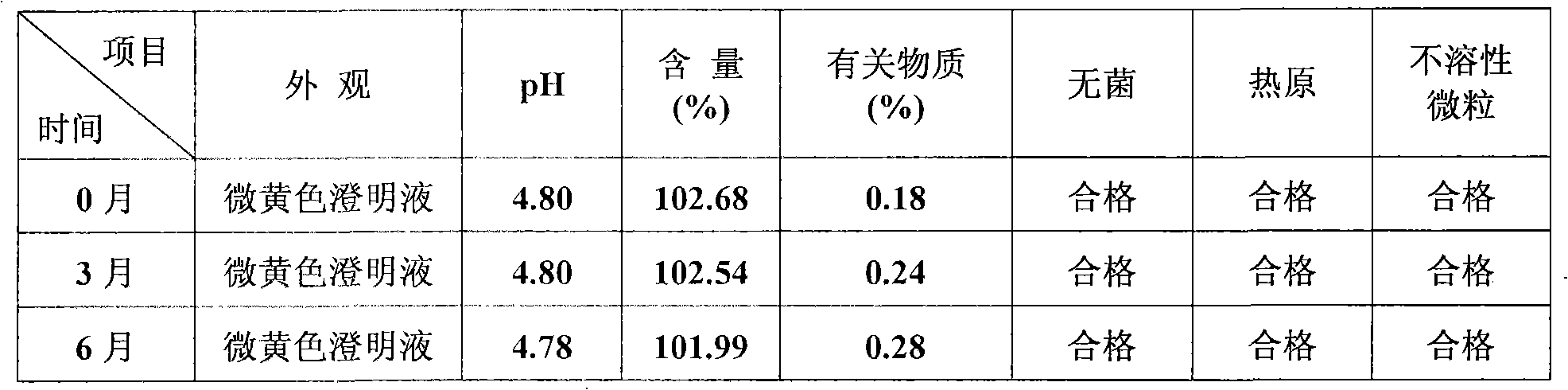
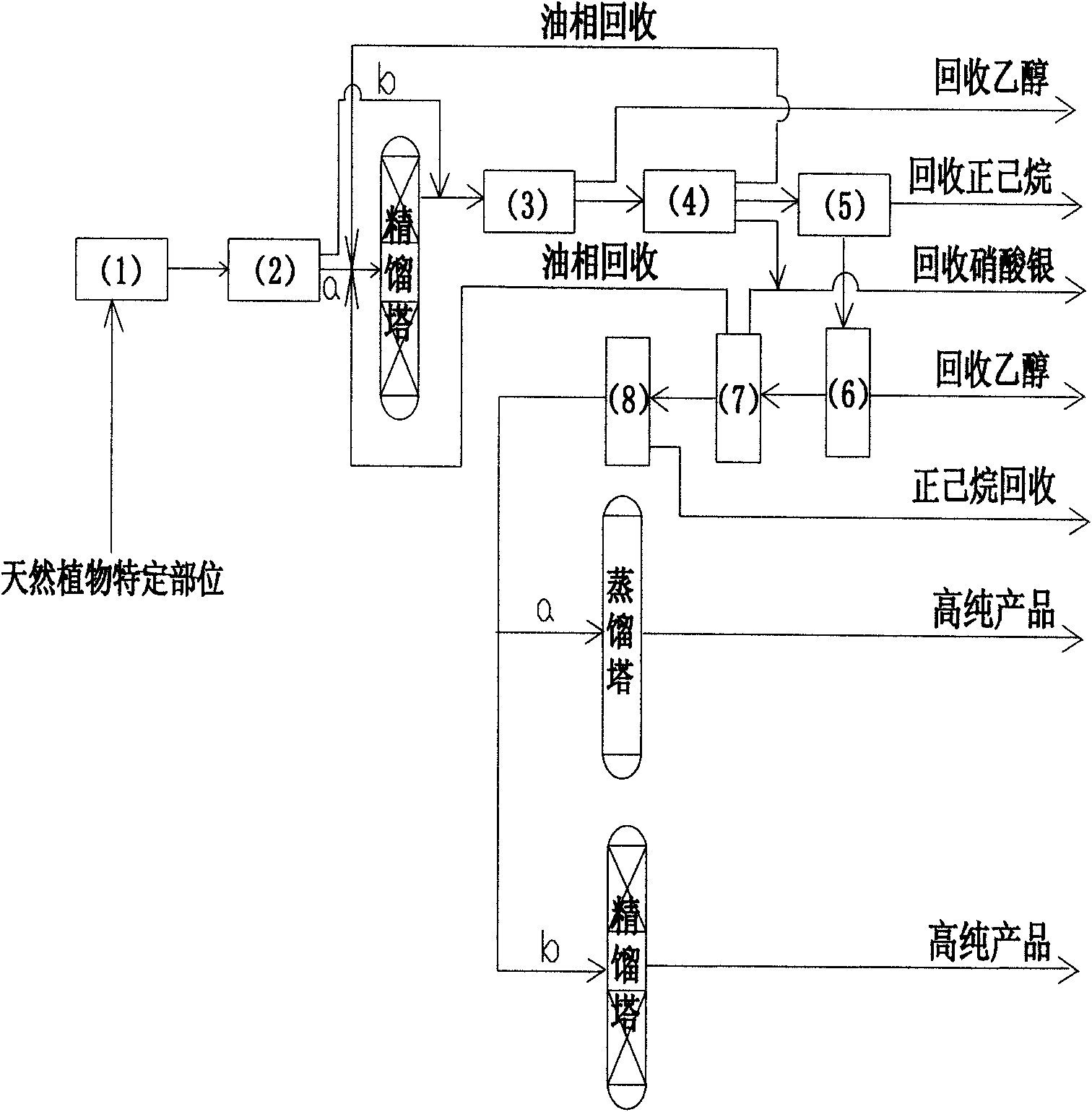
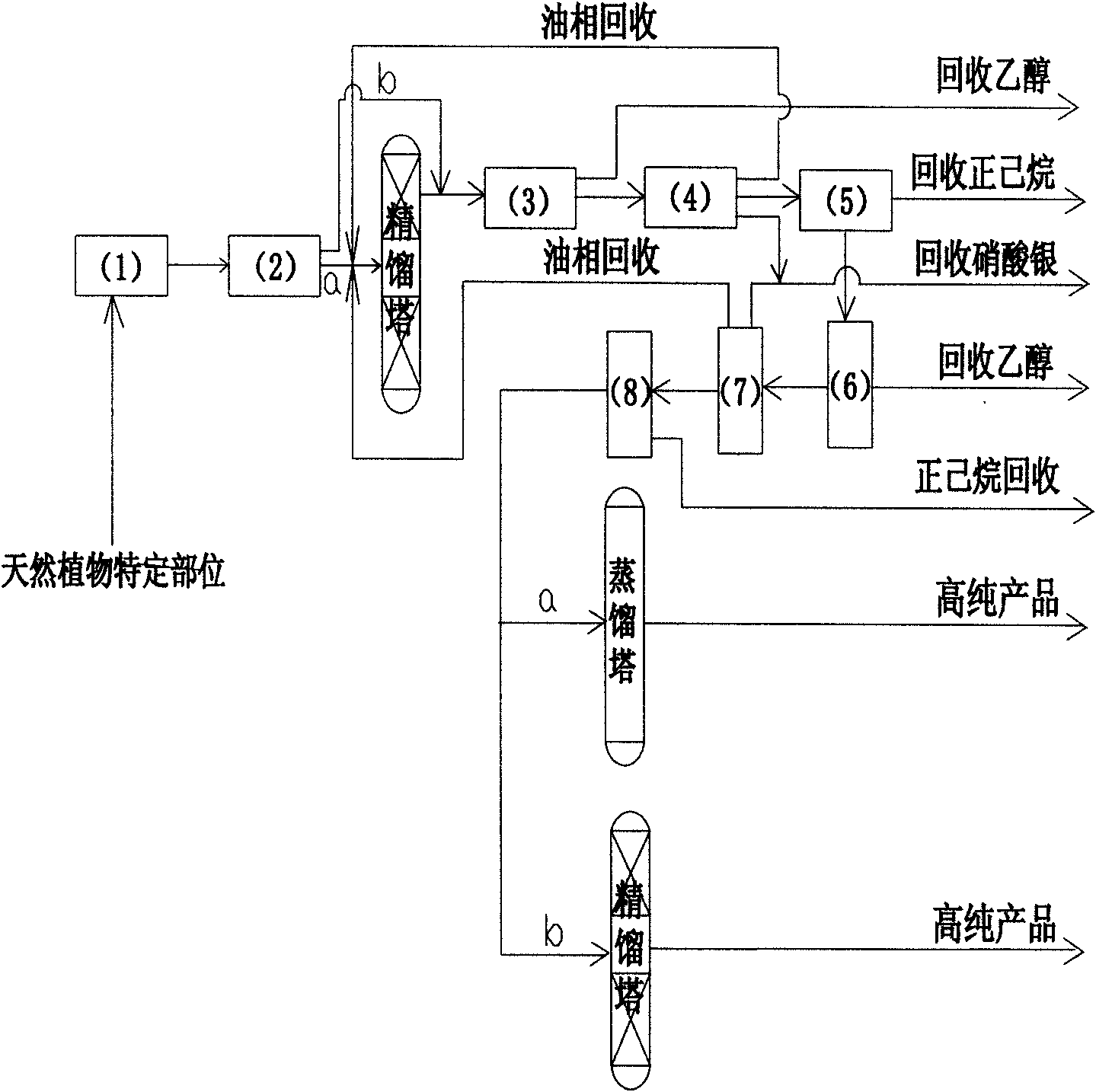
Method for simply preparing high-purity beta-Elemene
InactiveCN101613249AHydrocarbon active ingredientsDistillation purification/separationDistillationParenteral solutions
The invention provides a method for preparing high-purity beta-Elemene from natural plants containing beta-Elemene such as curcuma zedoary (root tubers or stem tubers of the curcuma zedoary), Cymbopogon citratus (fresh leaves of the Cymbopogon citrates), Solidago virgaurea (roots, stems, leaves, flowers and seeds of the Solidago virgaurea) and the like so as to improve production efficiency from the initial raw materials to the high-purity beta-Elemene and reduce production cost. Compared with the prior art, the method is mainly characterized in that: the roots, stems, leaves, flowers and seeds of the natural plants are taken as raw materials, and volatile oil of specific parts of the natural plants is prepared by methods for extracting different volatile oil, and then is rectified by a rectification method to prepare high-content beta-Elemene; then impurity components are removed by an ethanol extraction method and a silver nitrate complexing extraction method; finally the beta-Elemene with the content between 95.0 and 99.9 percent is prepared by distillation under reduced pressure; and the beta-Elemene not only can be prepared into oral administrative preparations such as emulsion oral liquid, capsules and the like, but also can be prepared into parenteral administrative preparations such as emulsion parenteral solution, injection, cutaneous permeable agents, lung spray preparations, suppositories and the like. The method has novel design of experimental methods, simple instruments and equipment, concise operation steps and obviously shortened operation time, improves production efficiency and is suitable for industrial production.
Owner:沈阳万爱普利德医药科技有限公司
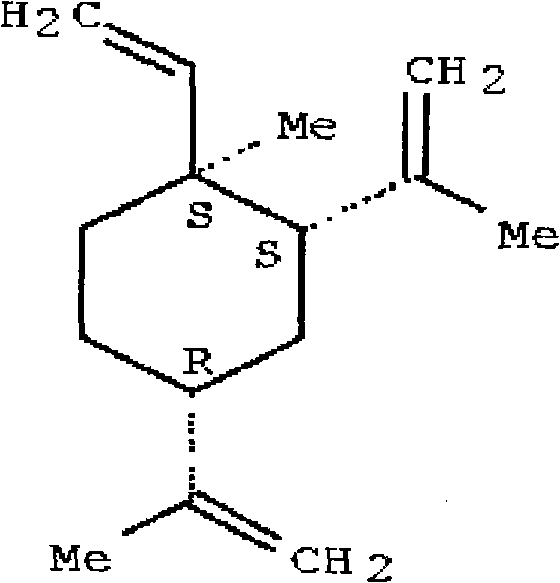
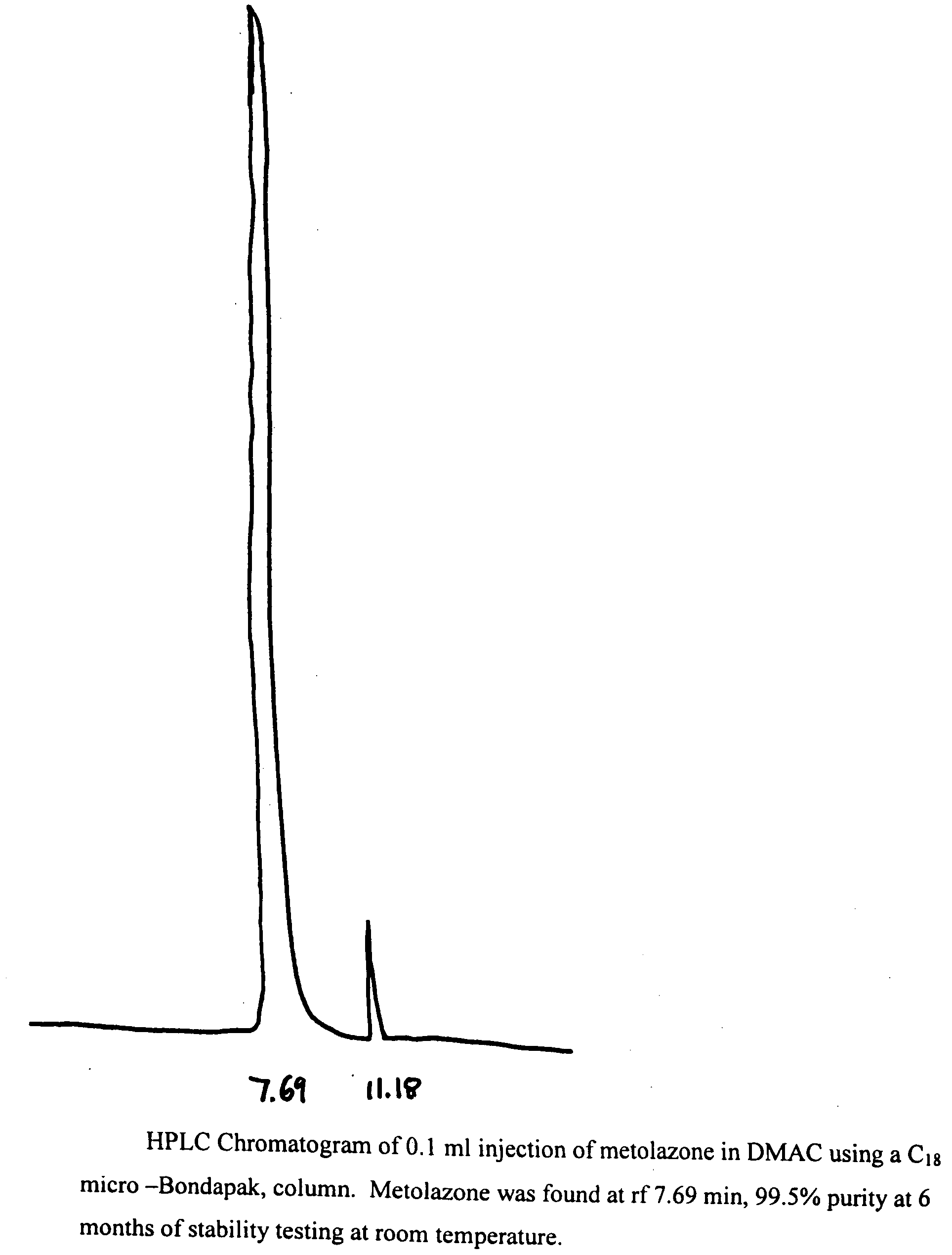
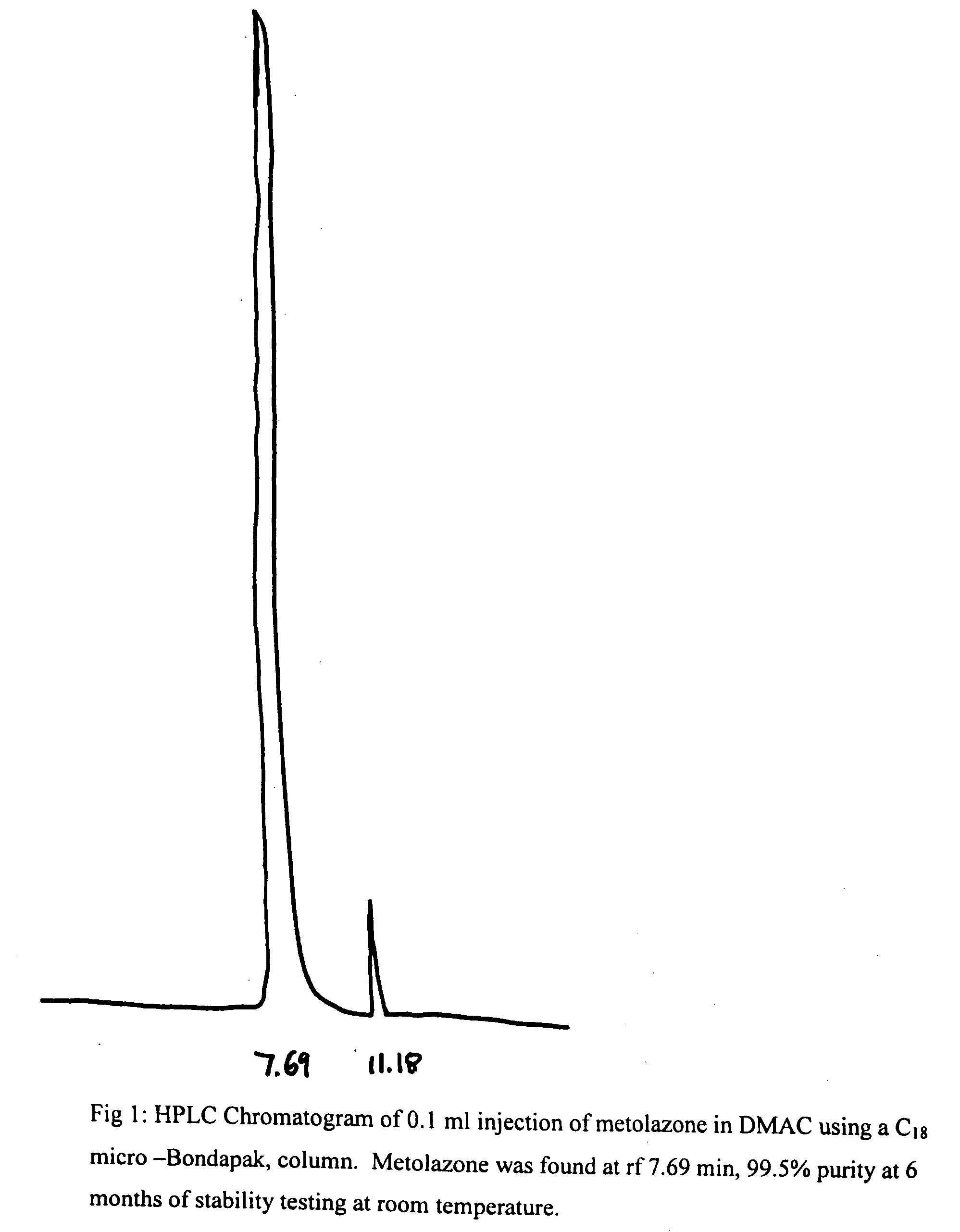
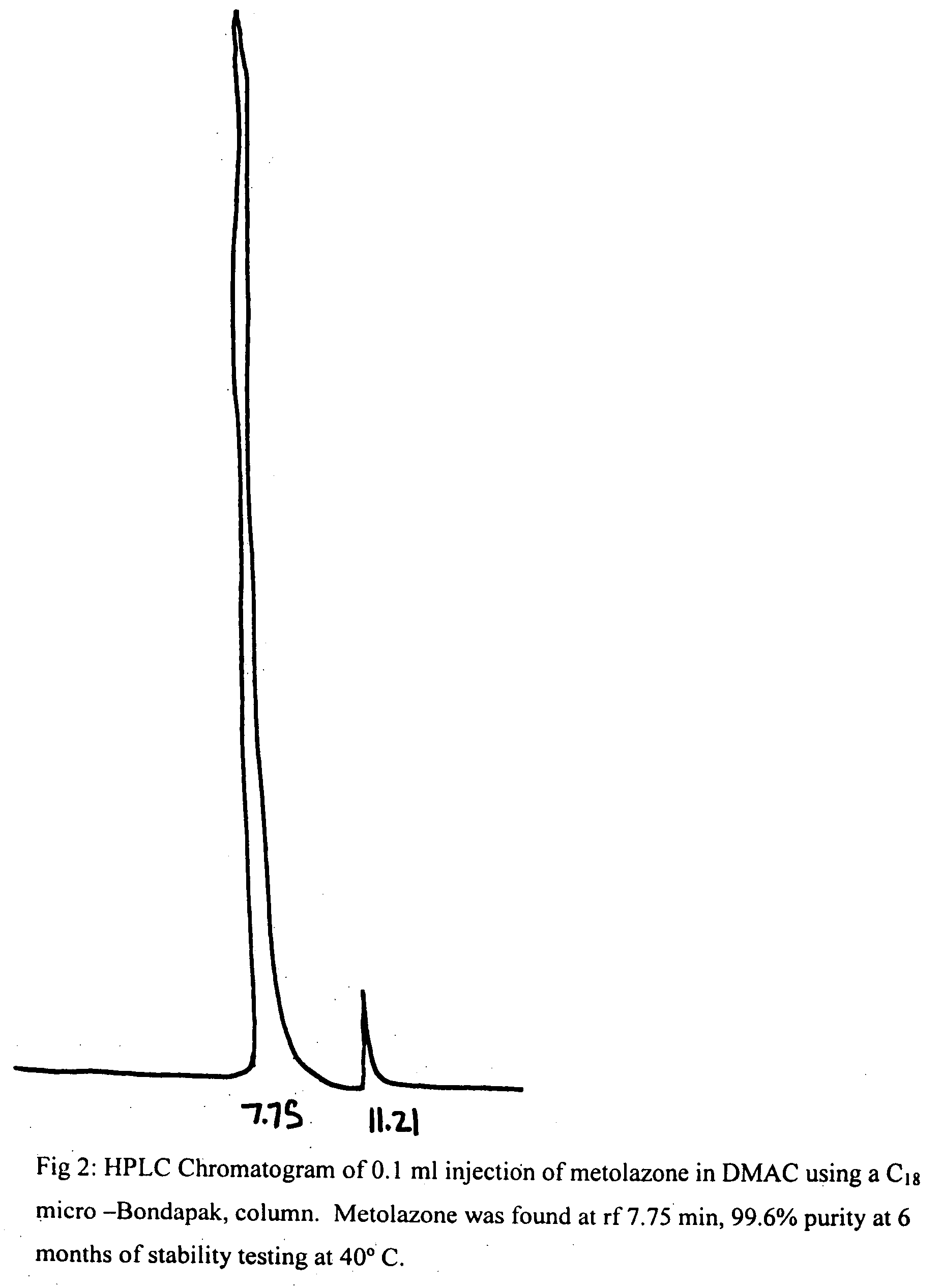
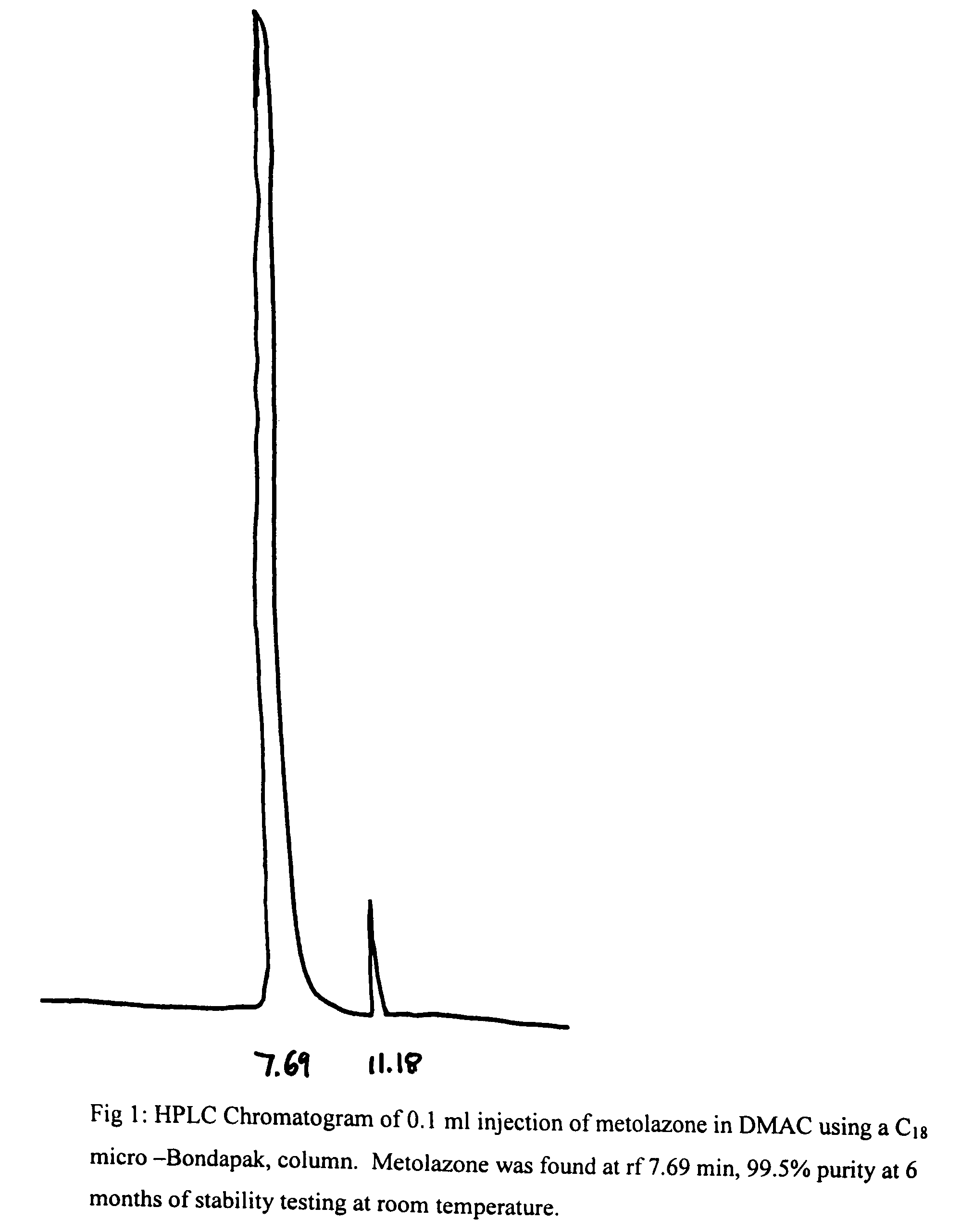
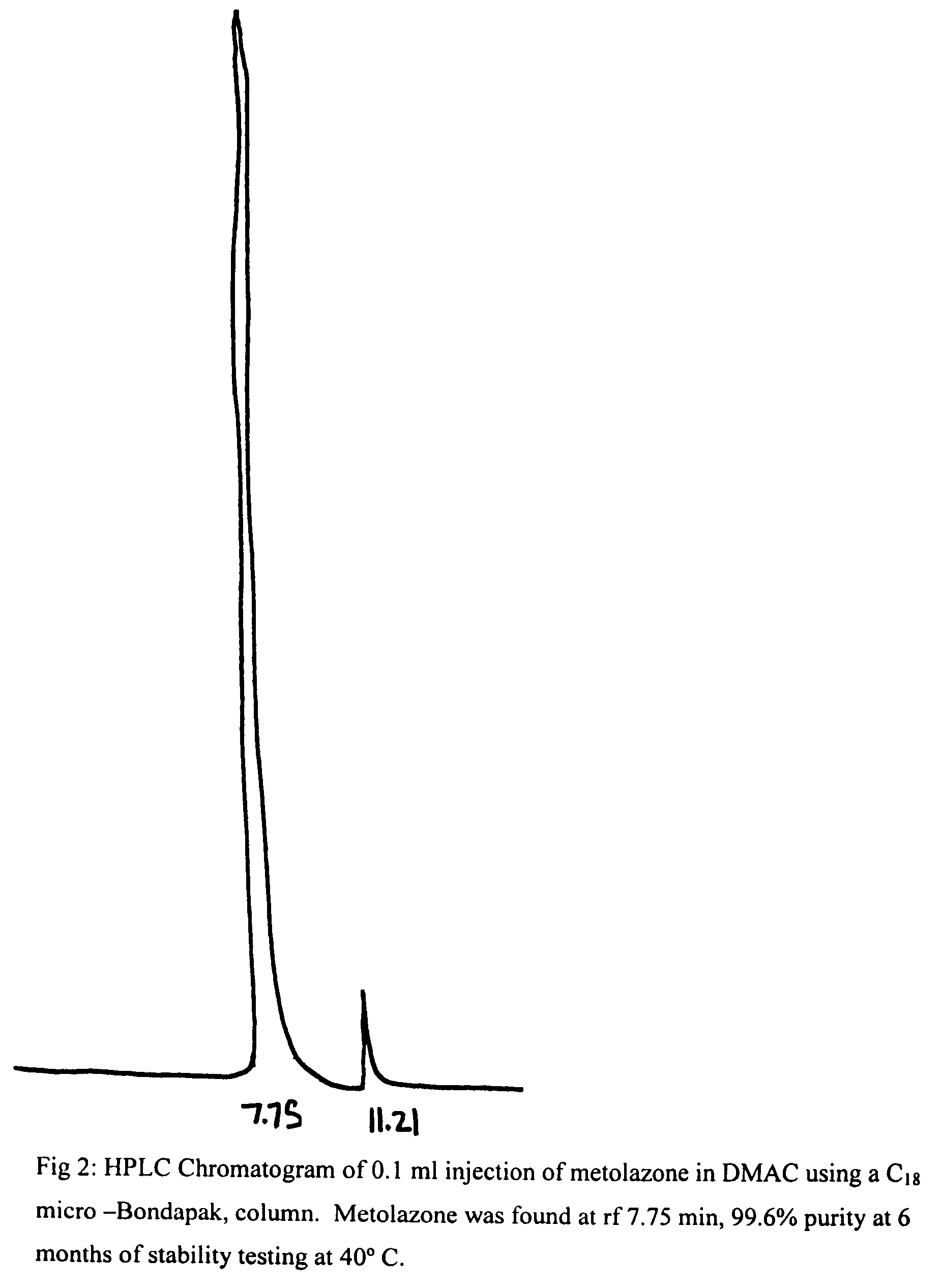
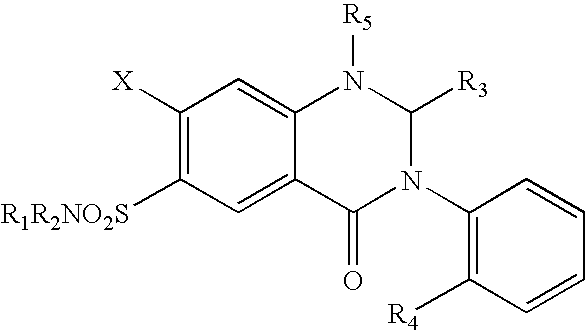
Parenteral solutions containing metolazone
ActiveUS20090233951A1Increase excretionIncrease volumeBiocidePharmaceutical delivery mechanismDiseaseAryl
Disclosed herein are parenteral solutions containing 7-halo-1,2,3,4-tetrahydro-3-aryl-6-quinazoline sulfonamide in N,N-dimethylactamide, polyethylene glycol and D5W useful in the treatment of hypertension, heart failure and renal disease leading to edematous states. Also disclosed are methods for preparing such solutions.
Owner:HYLORIS DEV SA
Composition containing glucosamine as well as preparation method and detection method thereof
ActiveCN102961389AMeet safety requirementsMeet stability requirementsOrganic active ingredientsAntipyreticSolubilitySide effect
The invention relates to a composition containing glucosamine. The active ingredients of the composition are glucosamine or pharmaceutically acceptable salt thereof and lidocaine or pharmaceutically acceptable salt thereof, and the composition provided by the invention can be prepared into an injection, preferably a parenteral solution, more preferably a small-volume injection. The parenteral solution further comprises an antioxidant, a pH regulator and an alkaline solvent. The composition containing glucosamine provided by the invention can be used for overcoming the defects that freeze-drying preparation of glucosamine is long in freeze-drying time, high in energy consumption and not beneficial to production in the prior art and free-dying preparations in the same batch are different in the shape and solubility. Control on the stability of the parenteral solution and related substances is realized, and the side effect caused by degradation products is reduced, so that the safety and stability requirements of the parenteral solution are met. Therefore, the glucosamine parenteral solution provided by the invention can meet industrial production and can be safely and reliably applied to the field of medicine.
Owner:任金山 +1
Injection preparation containing argatroban
InactiveCN102240393ADipeptide ingredientsPharmaceutical delivery mechanismLarge volume parenteralActive component
The invention relates to an injection preparation which adopts argatroban as an active component. The injection preparation is characterized by: adopting a single optical isomer of the argatroban or a salt of the argatroban or a hydrate of the argatroban as the medicinal active component and adding a plurality of auxiliary materials with specific types and specific ratios to prepare and develop the preparation for the intravenous injection according to a technical means described in the present patent specification. The formulations of the injection preparation comprise powder injection, small volume parenteral solution or large volume parenteral solution.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Method for preparing ceftiofur hydrochloride parenteral solution
InactiveCN101601645ASolve the difficulty of injectionSolve for uniformityAntibacterial agentsOrganic active ingredientsCeftiofur HydrochlorideParenteral solutions
The invention relates to a method for preparing ceftiofur hydrochloride parenteral solution; the hydrochloride parenteral solution has the characteristics of even distribution and clear and transparent solution. The method comprises the following steps: (1) adding propylene glycol and ceftiofur hydrochloride with a volume equal to 60-70% of the total volume of ceftiofur hydrochloride parenteral solution to be prepared in a thick mixing tank; adding 2-10g ceftiofur hydrochloride for every 100ml of total volume of the ceftiofur hydrochloride parenteral solution; heating to 40-50 DEG C and stirring for rapid dissolving; (2) adding propylene glycol to reach the total volume of the ceftiofur hydrochloride parenteral solution to be prepared and cooling to 20-30 DEG C; (3) adding active carbon, stirring for 15-20 minutes and filtering; and (4) filling and sealing, sterilizing, light check and packing. The invention solves the problems of difficult and uneven injection of the ceftiofur hydrochloride parenteral solution of the existing technology and makes up the defects of easy-layer and inconvenient use of the ceftiofur hydrochloride suspension injection of the existing technology.
Owner:上海恒丰强生物技术有限公司
C-acetate preparation method
InactiveCN101597228AReduce manufacturing costPreparation from carboxylic acid halideRadioactive preparation carriersBromineChloride
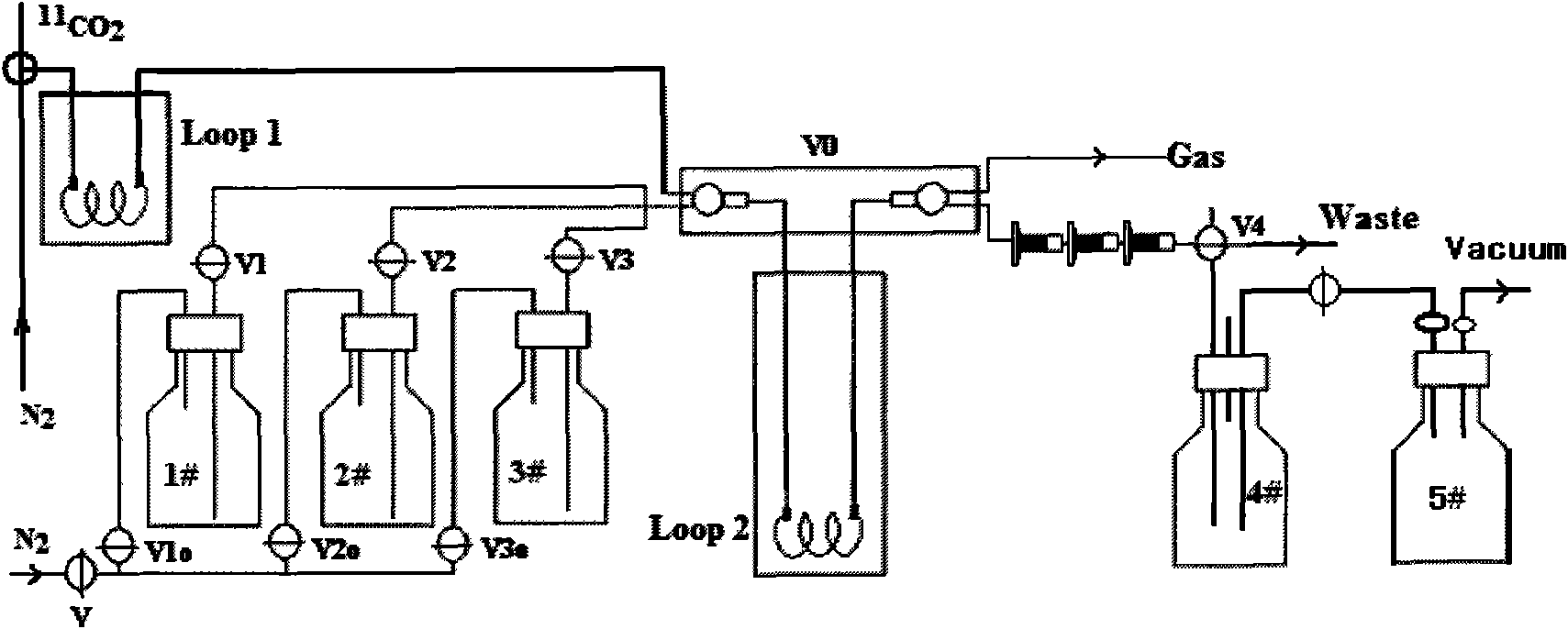
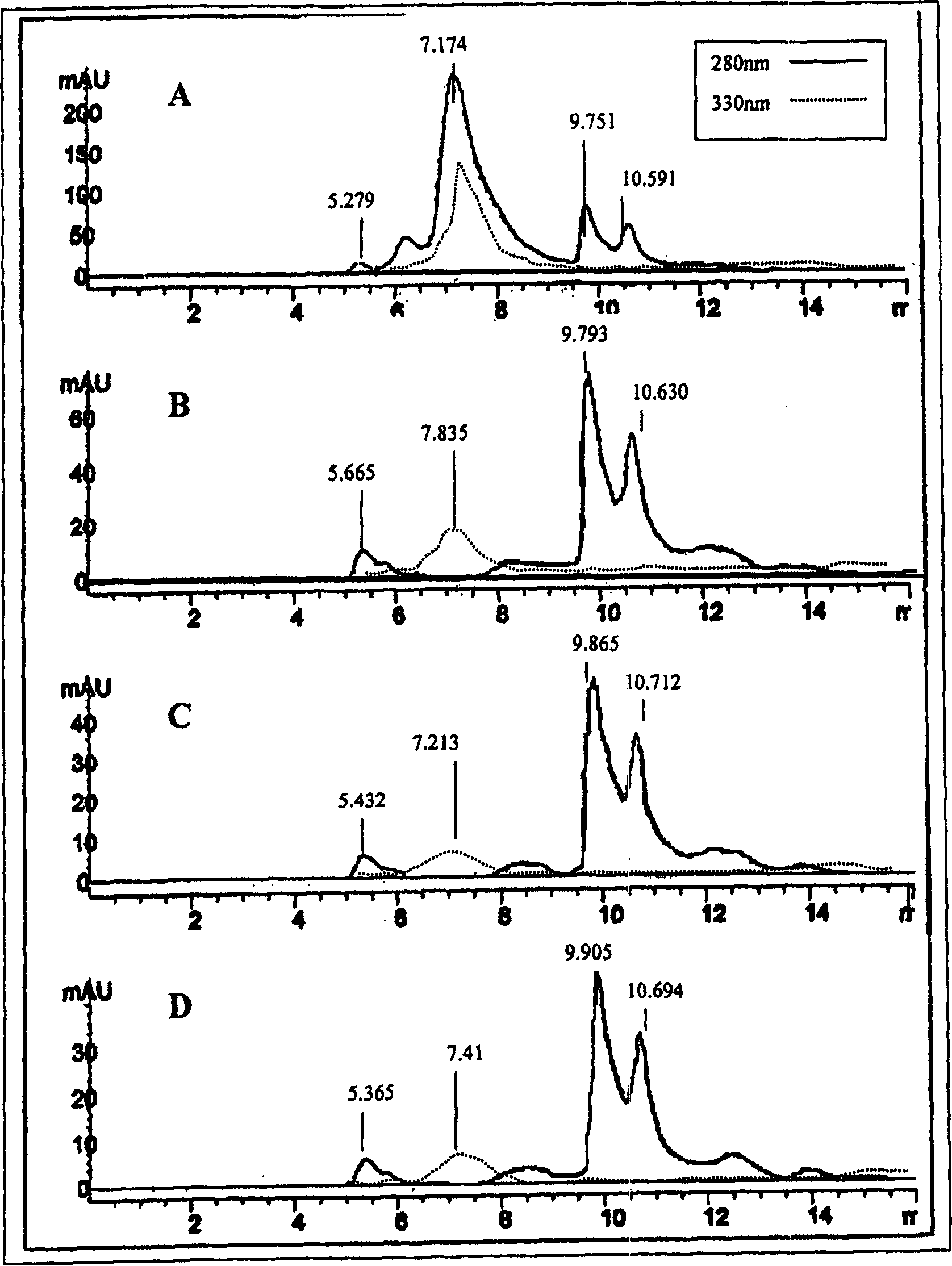
An C-acetate preparation method comprises the following steps: adopting methyl magnesium bromide or methyl magnesium chloride as precursor, reacting the precursor with CO2 in a Loop to prepare an intermediate C-acetyl bromine or magnesium chloride adduct; without adopting purification process, injecting N2 and removing tetrahydrofuran (THF), adding water in the mixture, injecting the mixture through integral small columns (C18 small column, SEP-PAK TSCX small column and SEP-PAK TIX small column) in turn for isolation and purification where the intermediate C-acetyl bromine or magnesium chloride hydrolyzes, filtrating by aseptic filter membrane, neutralizing by alkaline solution and obtaining C-AC parenteral solution. The preparation method of the invention has the advantages of the realization of column hydrolysis and purification, fully automatic synthesis, short synthesis time, high radiochemical yield, high radiochemical purity and low production cost.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
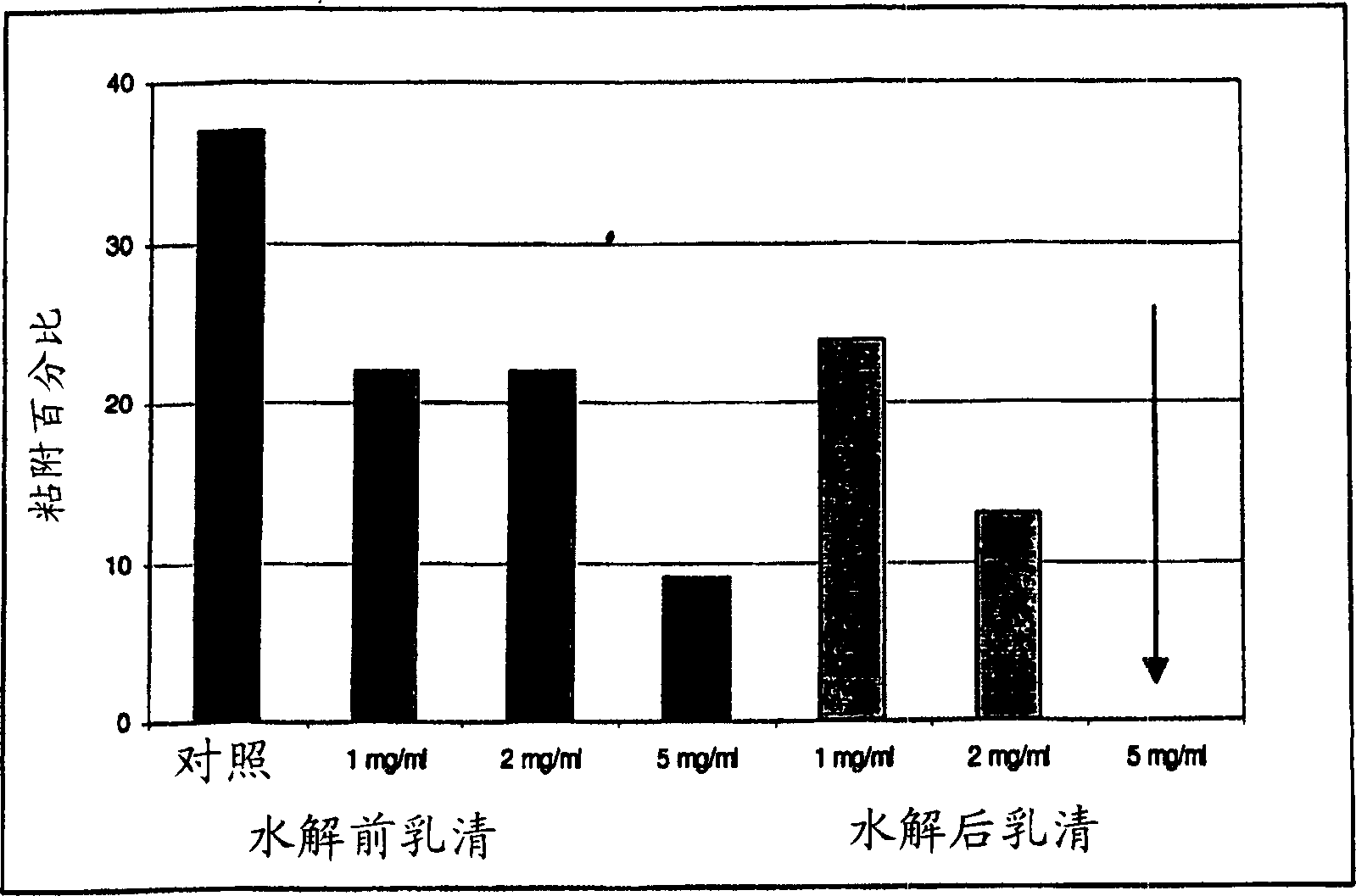
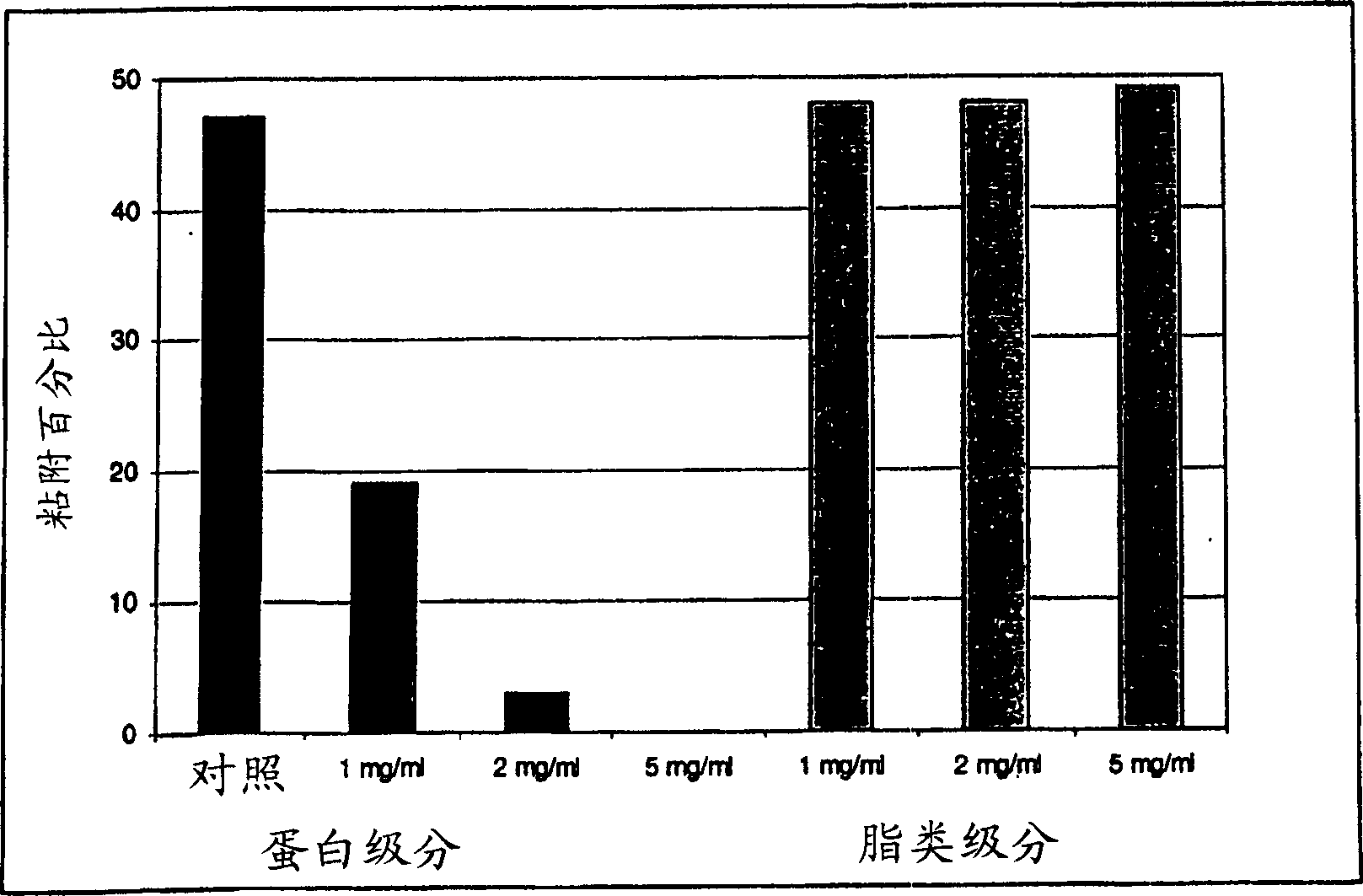
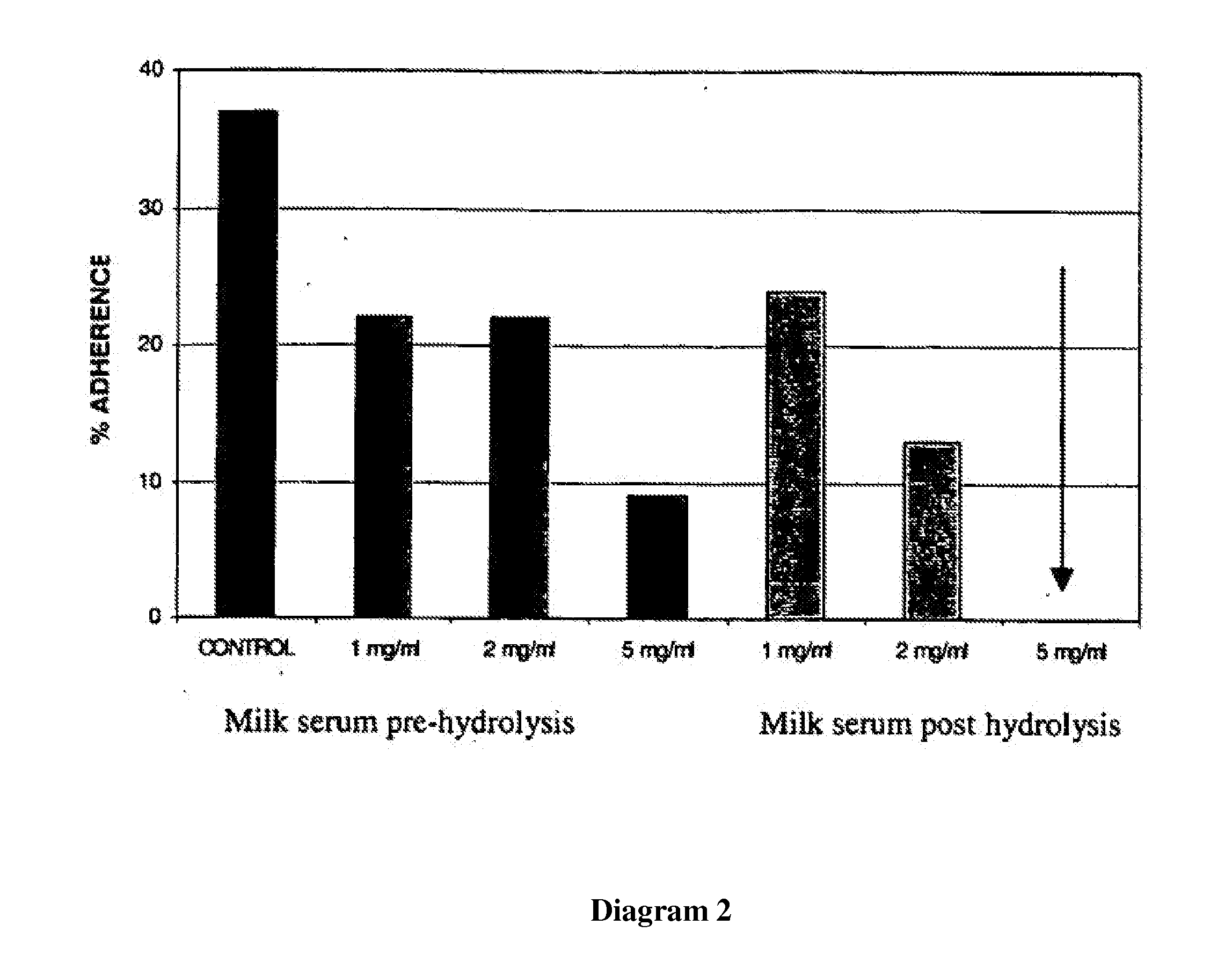
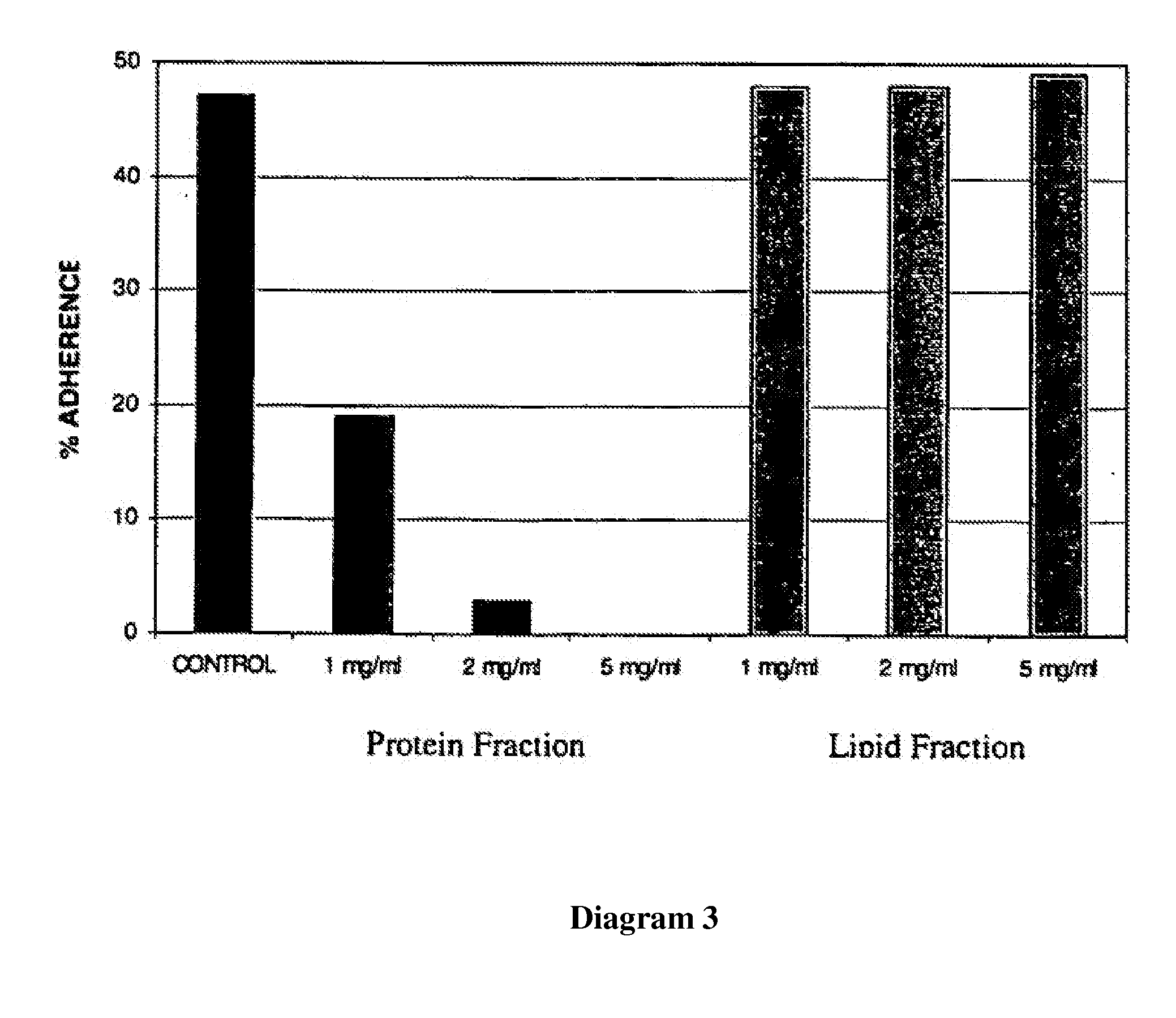
Use of lactalapoprotein in prevention and treatment of microbial or virus infection
The present invention relates to use of a milk apoprotein or a mixture thereof to prevent or treat microbial or viral infection of the human or animal body. It is believed that this is achieved by inhibiting adhesion of potential pathogens. More preferably, at least one milk apoprotein or a mixture thereof is administered, simultaneously or sequentially, with either or both of at least one free fatty acid or a mixture thereof or a monoglyceride thereof; and / or at least one organic acid or a salt or ester thereof or a mixture thereof. The active agent(s) may be delivered by means of a pharmaceutically acceptable delivery system which includes parenteral solutions, ointments, eye drops, nasal sprays, intravaginal devices, surgical dressings, medical foods or drinks, oral healthcare formulations and medicaments for mucosal applications.
Owner:WESTGATE BIOLOGICAL
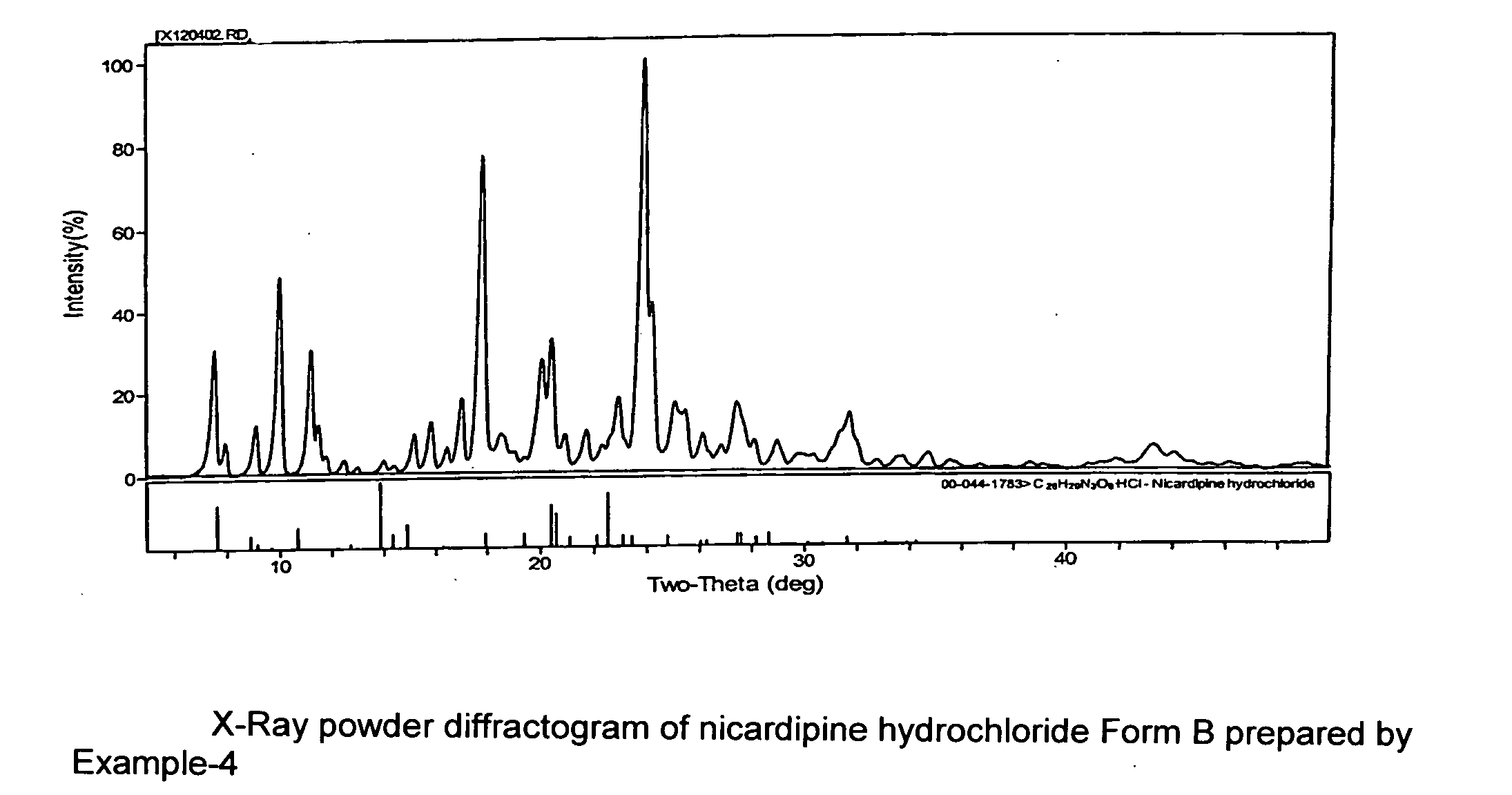
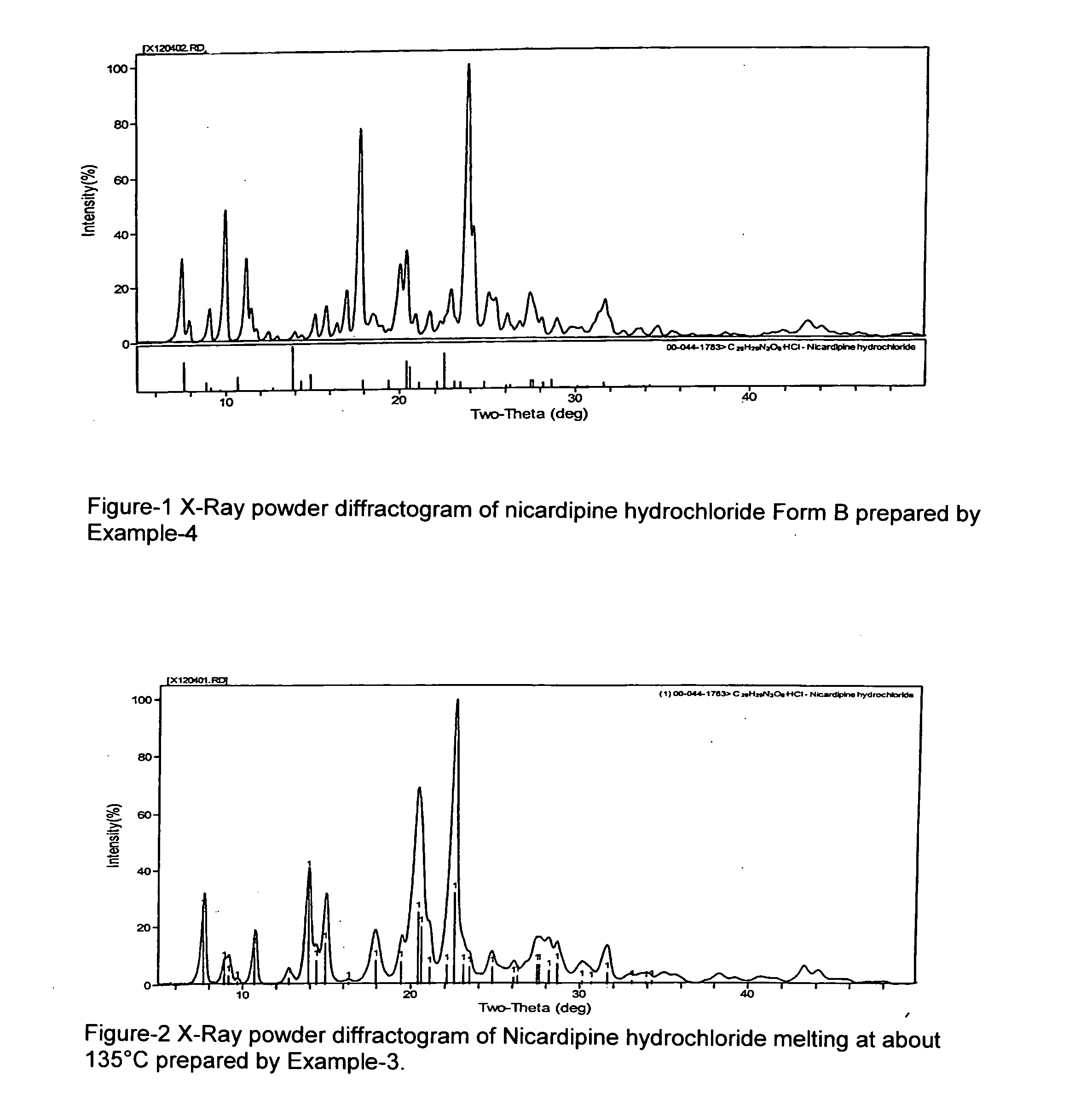
Processes of manufacturing substituted-1,4-dihydropyridines, improved aqueous solutions thereof, and processes of manufacturing the solutions
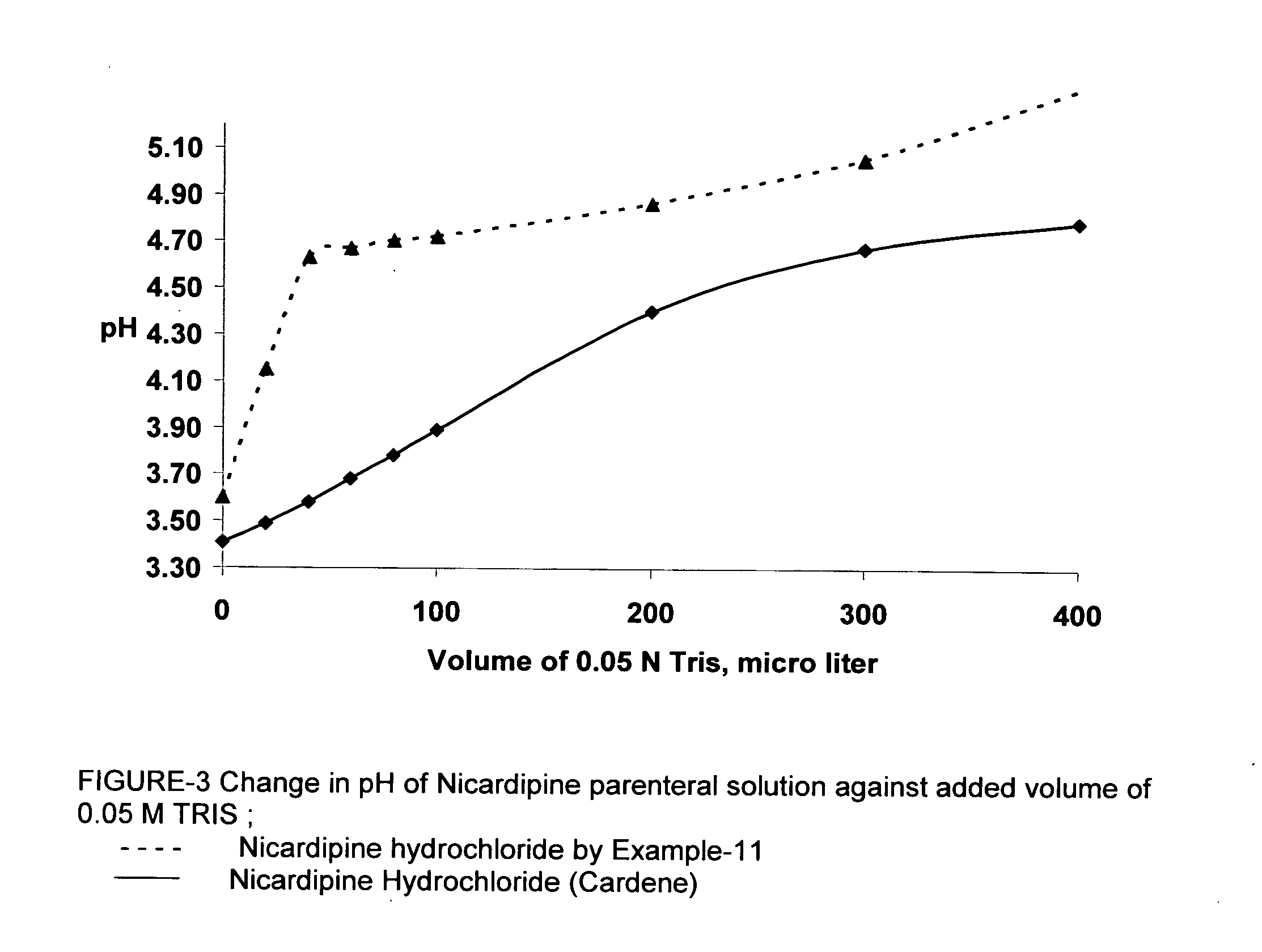
A process of preparing a stable parenteral solution of a 1,4-dihydropyridine salt, such as nicardipine hydrochloride, in an acidic aqueous medium. The presence of L-arginine in the solution enhances the solubility of the salt, which is poorly soluble in water.An aqueous, injectable isotonic solution at pH about 3.5-3.6 consists essentially of nicardipine hydrochloride, L-arginine, and a sugar alcohol.An improved single pot manufacturing process for obtaining unsymmetrical 1,4-dihydropyridines by using more than one mole equivalent of aldehyde with respect to the other reactants (amino crotonate and acetoacetate ester). The reaction can be conducted in a solvent present at 20 times the amount of any one component.A process for changing one polymorph of nicardipine hydrochloride (Form A) into another (Form B), and a separate process for the reverse (Form B into Form A).
Owner:NAVINTA
Traditional Chinese medicine injection for treating pig fever symptom and preparation thereof
The invention discloses a Chinese materia medica parenteral solution for the treatment of swine fever disease and its preparation methods; it is a kind of pharmaceutical product made of raw materials based on the following weight ratio: heartleaf houttuynia herb 2-6 portions, wild chrysanthemum 2-6 portions, herba solidaginis 2-6 portions, caulis lonicerae 2-6 portions, Bupleurum chinense 0.5-1.5 portions. Its preparation methods is as follows, mix the Chinese materia medica raw materials heartleaf houttuynia herb, wild chrysanthemum, herba solidaginis, caulis lonicerae, Bupleurum chinense pro rate and then soak them, the ratio of the water and raw materials is 3-6:1, after the raw materials is drenched, distill them with the soak, then the physic liquor is got, then put the gruffs into the containers, add 95% alcohol solution the volume of which is twice by that of the gruffs and extract the effective drug component, finally, the injection of the said invention is got after the distilled physic liquor and the extraction physic liquor is mixed together, after sterilization and packing the products is obtained.
Owner:张玉柱
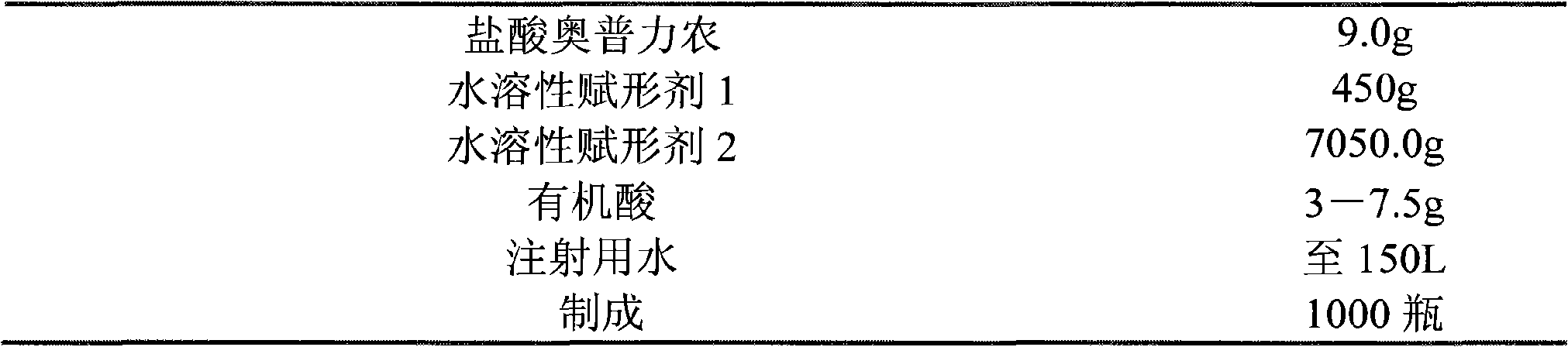
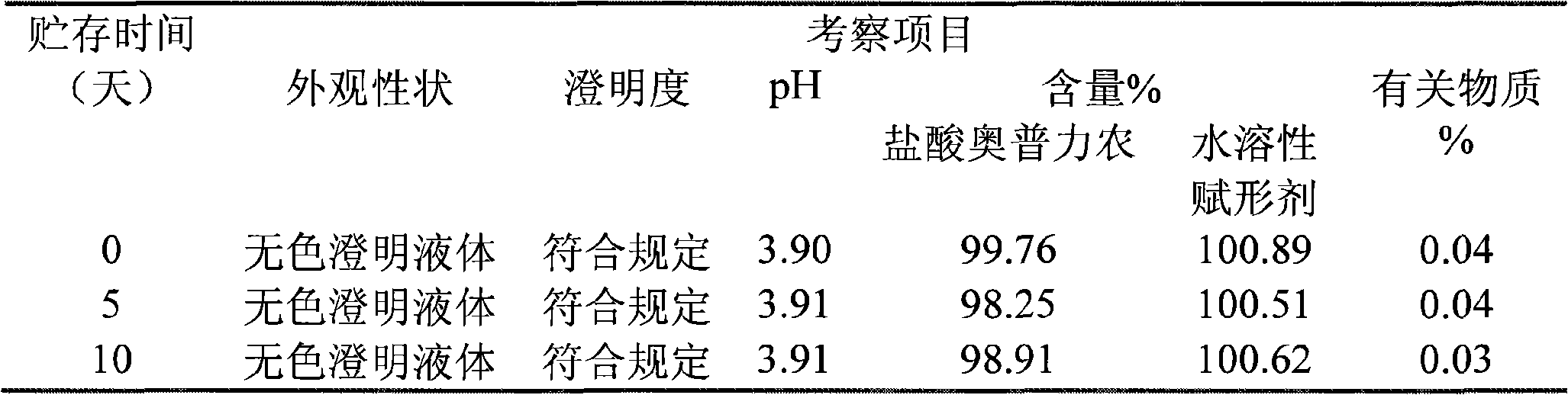
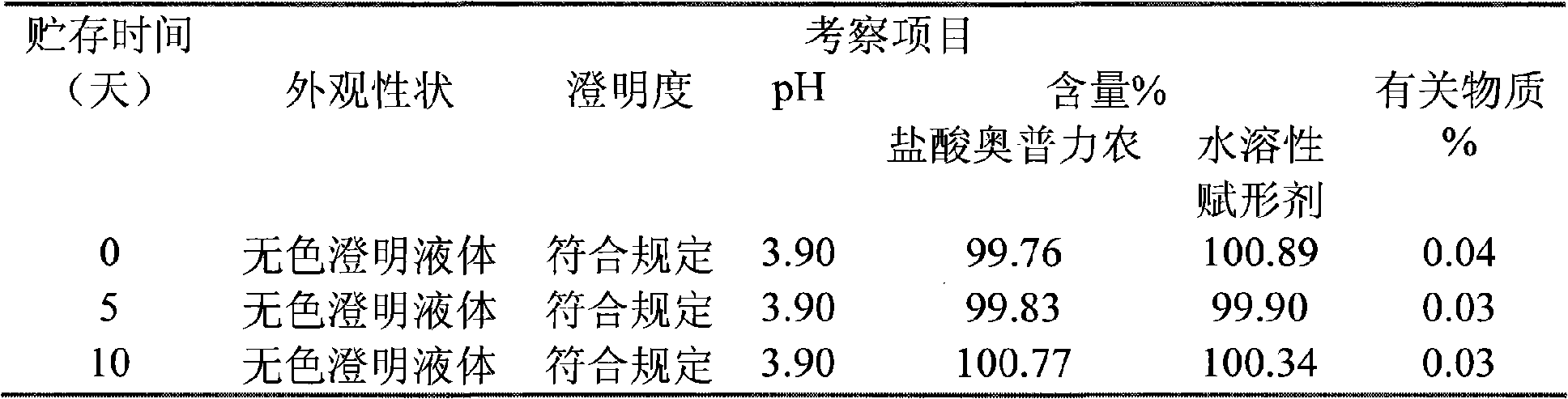
Olprinone hydrochloric parenteral solution and method for preparing same
The invention discloses olprinone hydrochloric parenteral solution and a method for preparing the same. In the parenteral solution, water-soluble excipient and organic acids serve as pharmaceutical adjuvant. In the parenteral solution in the invention, fewer sorts of pharmaceutical adjuvant are used, the dosage of the pharmaceutical adjuvant organic acids is greatly reduced, and on the basis of guaranteeing the quality of the preparation, the safety of the preparation is greatly improved.
Owner:西安万隆制药股份有限公司
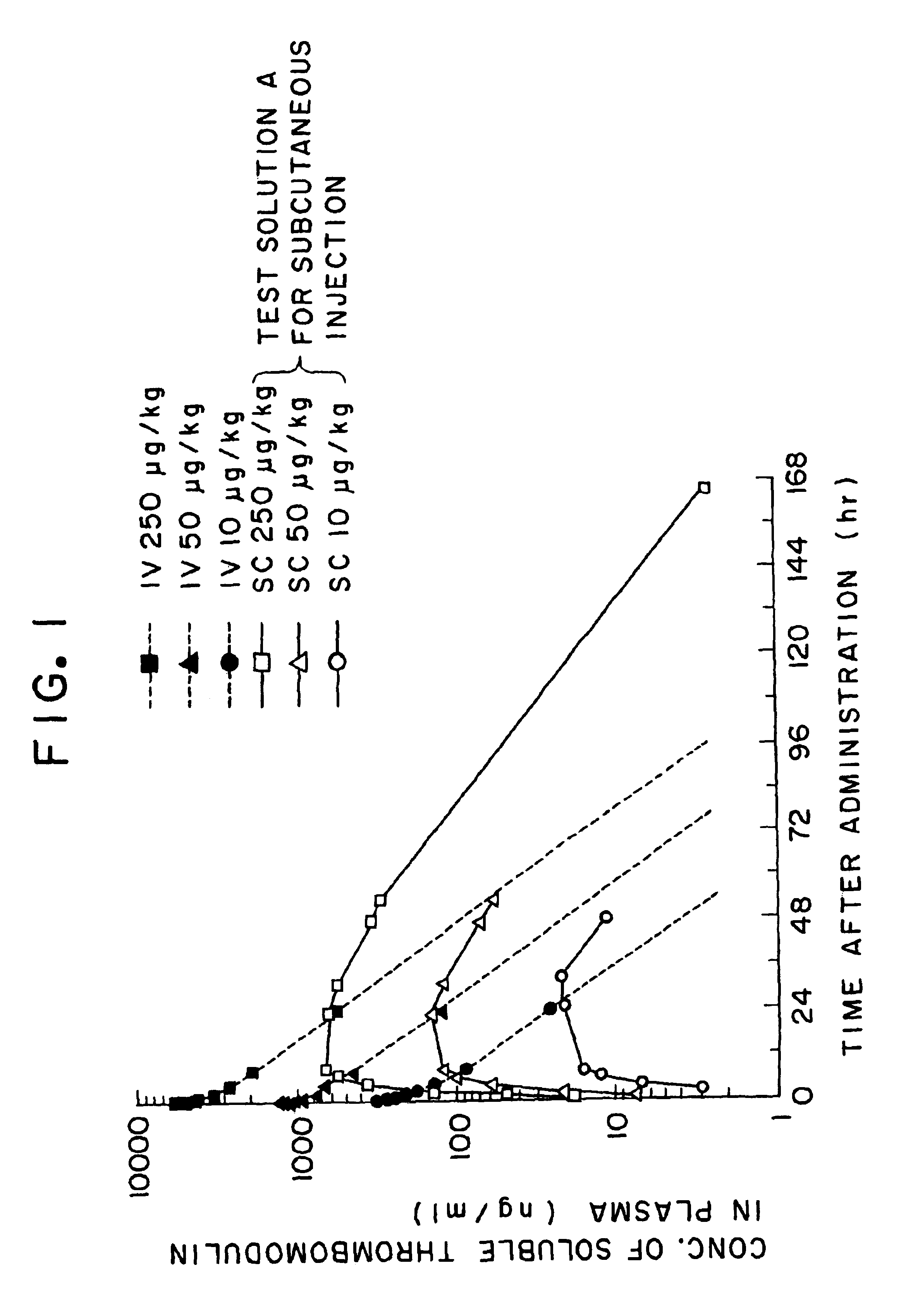
Method for keeping the quality of aqueous parenteral solution of thrombomodulin in storage and distribution
InactiveUS6808706B1ConvenientlyProduced economicallyPeptide/protein ingredientsPharmaceutical delivery mechanismFreeze-dryingLiquid state
A method for keeping the quality of an aqueous parenteral solution of thrombomodulin which is not in a frozen or freeze-dried state but in a liquid form in storage and distribution, characterized in that the aqueous thrombomodulin solution containing an effective amount of soluble thrombomodulin and a buffer component exhibiting a buffering activity in a pH range of 5 to 7.0 has a pH of 5 to 7.0 and that (a) the aqueous thrombomodulin solution further contains a surfactant and is in a state aseptically filled into a case or (b) the aqueous thrombomodulin solution is the form of a prefilled syringe preparation produced by aseptically filling the thrombomodulin solution into a syringe substantially without any empty space. This method provides for the storage and distribution of an aqueous parenteral solution of thrombomodulin in a liquid state for a prolonged period and makes it possible to provide an aqueous parenteral solution which is excellent in long-term stability and shaking stability and can save the trouble of dissolving in use.
Owner:ASAHI KASEI PHARMA
Product comprising a combination of milk serum apoproteins and free fatty acids
InactiveUS20100209526A1Potent and broad-spectrum inhibition of adhesionAvoid stickingAntibacterial agentsCosmetic preparationsMonoglycerideActive agent
The present invention relates to use of a milk apoprotein or a mixture thereof to prevent or treat microbial or viral infection of the human or animal body. It is believed that this is achieved by inhibiting adhesion of potential pathogens. More preferably, at least one milk apoprotein or a mixture thereof is administered, simultaneously or sequentially, with either or both of at least one free fatty acid or a mixture thereof or a monoglyceride thereof; and / or at least one organic acid or a salt or ester thereof or a mixture thereof. The active agent(s) may be delivered by means of a pharmaceutically acceptable delivery system which includes parenteral solutions, ointments, eye drops, nasal sprays, intravaginal devices, surgical dressings, medical foods or drinks, oral healthcare formulations and medicaments for mucosal applications.
Owner:WESTGATE BIOLOGICAL
Formulations of vancomycin
ActiveUS10376559B2Low viscosityImprove stabilityAntibacterial agentsOrganic active ingredientsPolyolGlycerol
Liquid vancomycin containing compositions having extended shelf life are disclosed. The compositions contain vancomycin or a pharmaceutically acceptable salt thereof, a polyol such as glycerol, and lactic acid or a lactate. The compositions are ready to use and easily transferred into larger parenteral solutions prior to administration to patients in need thereof.
Owner:SCIDOSE PHARMA LLC
Acehytisine freeze-drying emulsion and reparation method thereof
InactiveCN101716150ASolving IV problemsGive full play to the therapeutic effectPowder deliveryOrganic active ingredientsEmulsionFreeze-drying
The invention discloses an acehytisine freeze-drying emulsion and a reparation method thereof. The acehytisine freeze-drying emulsion uses the acehytisine as the active component, comprises oil for injection, emulsifier, additive and freeze-drying protective agent generally used in pharmacy and is dispersed into the emulsifier combination solution having a grain diameter ranging from 300 to 400nm after being added with water for injection. Compared with the acehytisine hydrochloride parenteral solution, the invention can realize 1.36 times of AUC and 1.59 times of MRT with half dosage and has equivalent pesticide effect for resisting arrhythmia.
Owner:刘静涵 +3
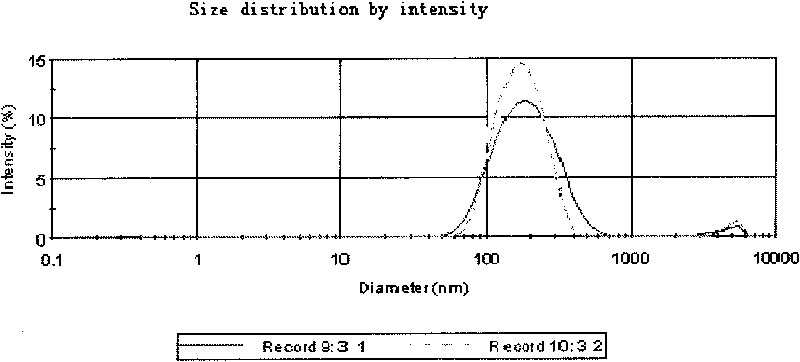
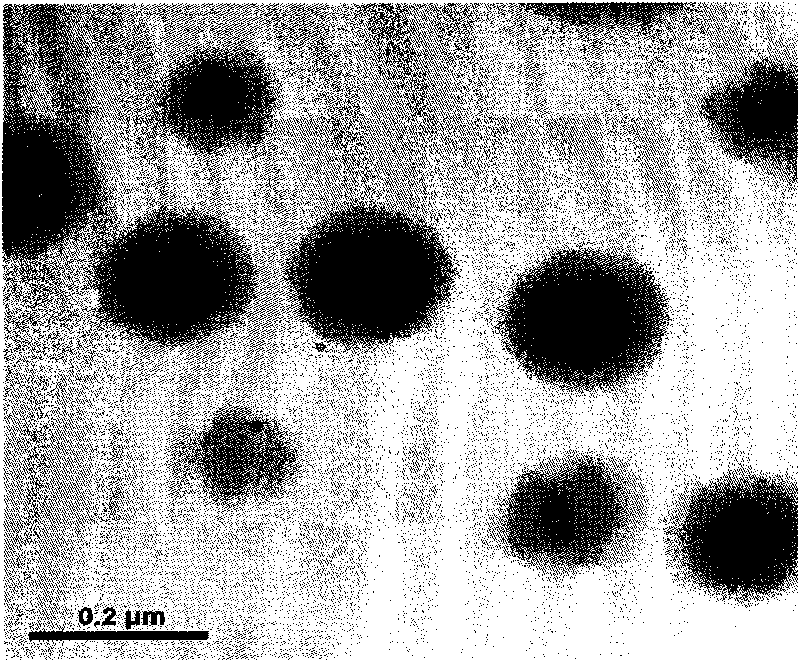
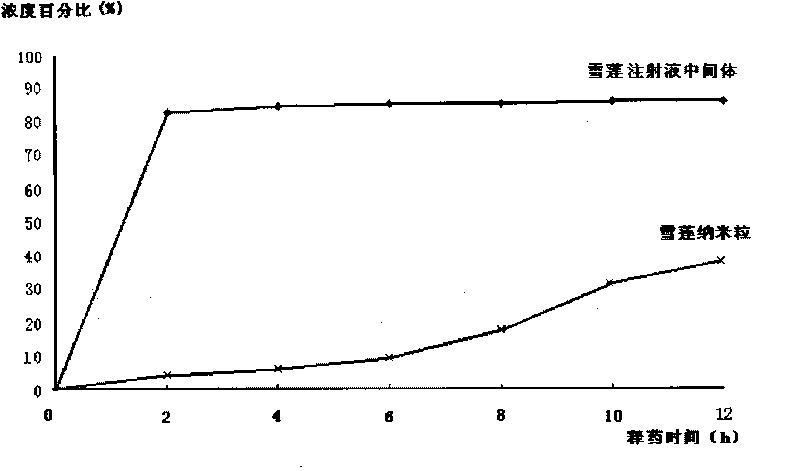
Saussurea involucrate nano particles and preparation method and application thereof
The invention discloses saussurea involucrate nano particles and a preparation method and an application of the prepared saussurea involucrate nano particles. The saussurea involucrate nano particles in the invention is prepared by the following steps: taking oil phase W emulsified particles as the carrier, taking the aqueous solution of the effective component in the saussurea involucrate or the alcohol solvent O as the internal aqueous phase carrying on the oil phase W emulsified particles by stirring at a high speed to form W / O particles, and coating a covering layer of emulsifier outside the W / O particles to form W / O / W multiple emulsion nano particles. Measurements and tests prove that the grain diameter of the saussurea involucrate nano particles in the invention is in a range of 60-800 nm, the average grain diameter is 134.6nm; saussurea involucrate nano particles in the invention have a shape of quasisphere, smooth surface, even size, no bond between particles, high drug-loading rate, good encapsulating rate and releasing effect and stable releasing rate, can be prepared into oral liquid, tablets, various capsules such as soft capsules, hard capsules and microcapsules, and can be prepared into parenteral solution, lotion, embrocation, patch or electuary.
Owner:SINOPHARM GRP XINJIANG PHARMA
Complex traditional Chinese medicine preparation for treating myocardial ischemia and improving microcirculation and preparing method thereof
The invention relates to a Chinese traditional compound medicine and the preparative way of it, which is used to treat myocardial ischemia and improve the microcirculation. The medicine is made up with American ginseng and ophiopogonis and the main ingredients are American ginseng diol group saponin, para-panaxsaponin F11, ophiopogonis Steroid saponin and ophiopogonone, of which American ginseng diol group saponin can't be less than 14.848g, while the American ginseng three groups saponin can't be less than 8.016g. The medicine can be made into different dosage forms such as parenteral solution, high-capacity parenteral solution, freeze-drying powder ampul and so on. The administration route is through intravenous injection.
Owner:刘玉辉
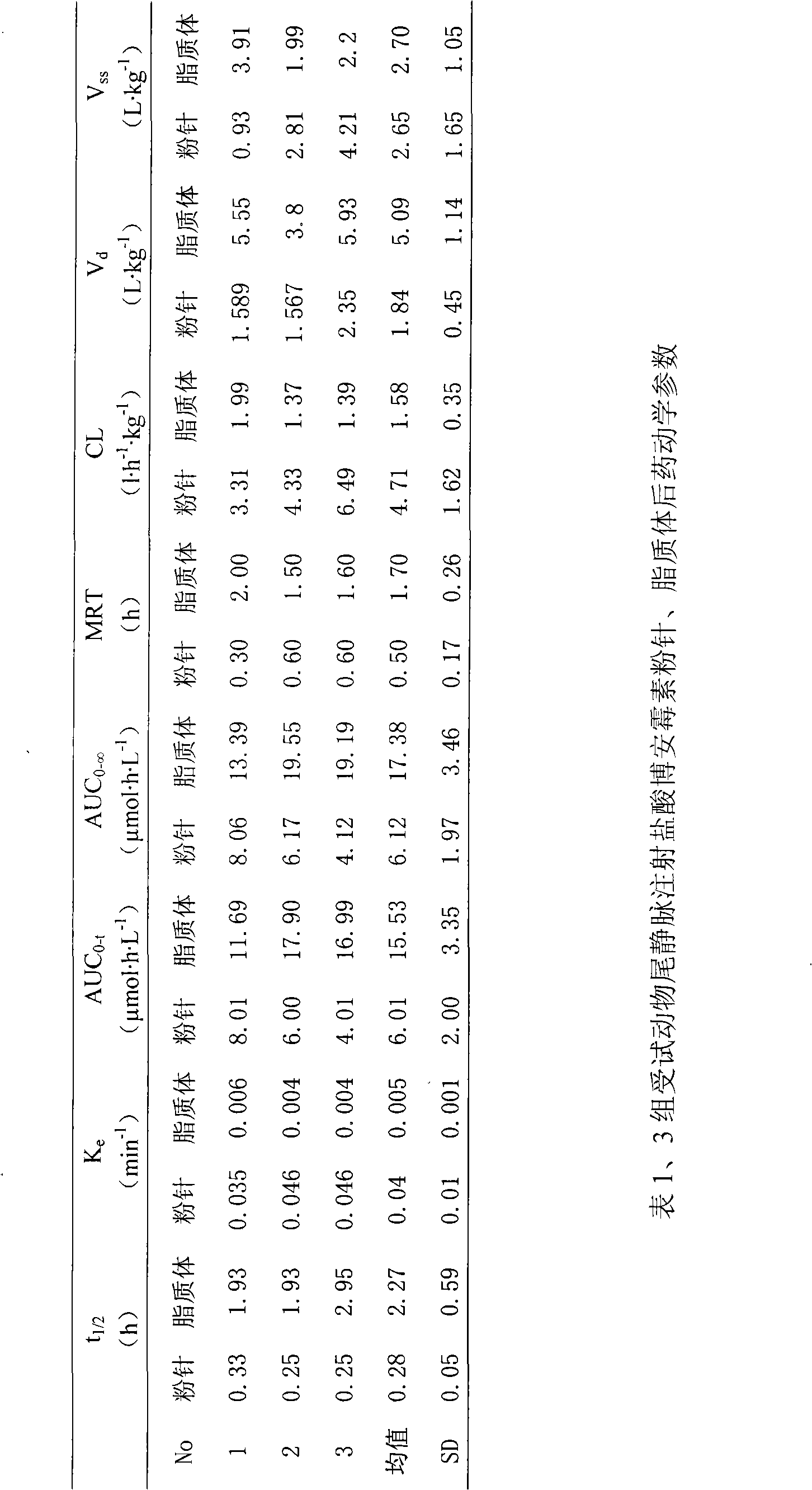
Hydrochloric acid boanmycin liposomes and preparation method thereof
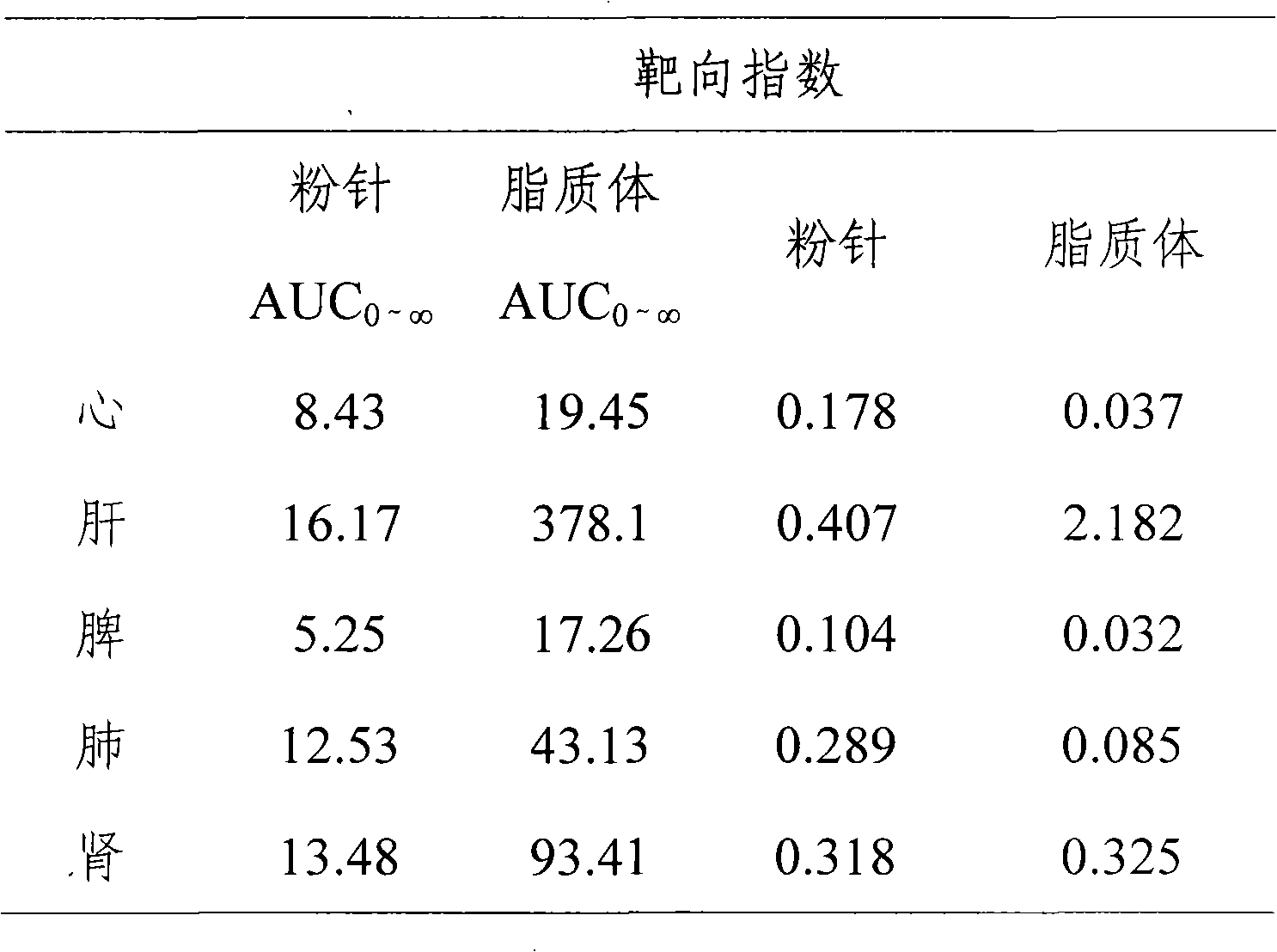
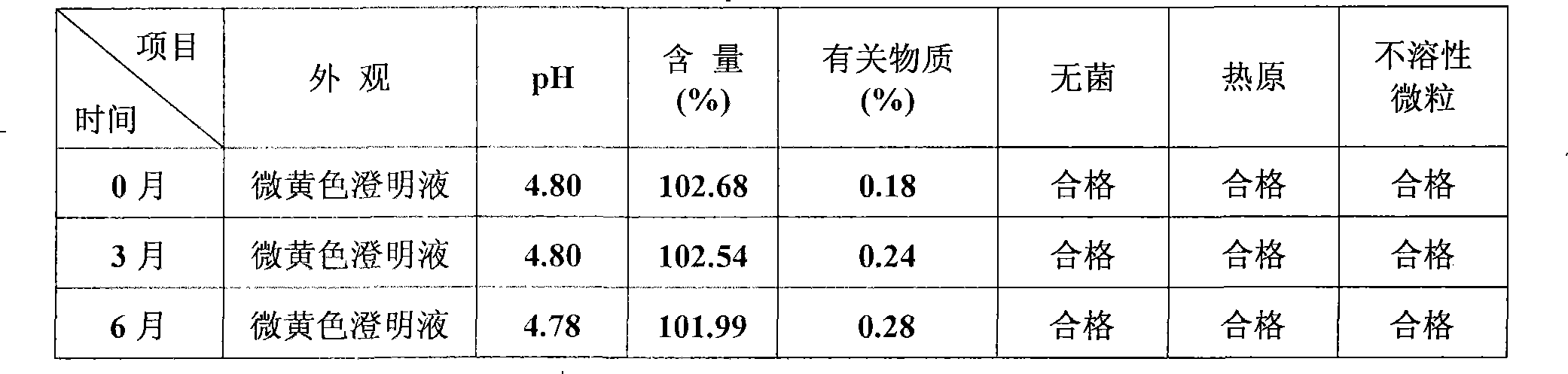
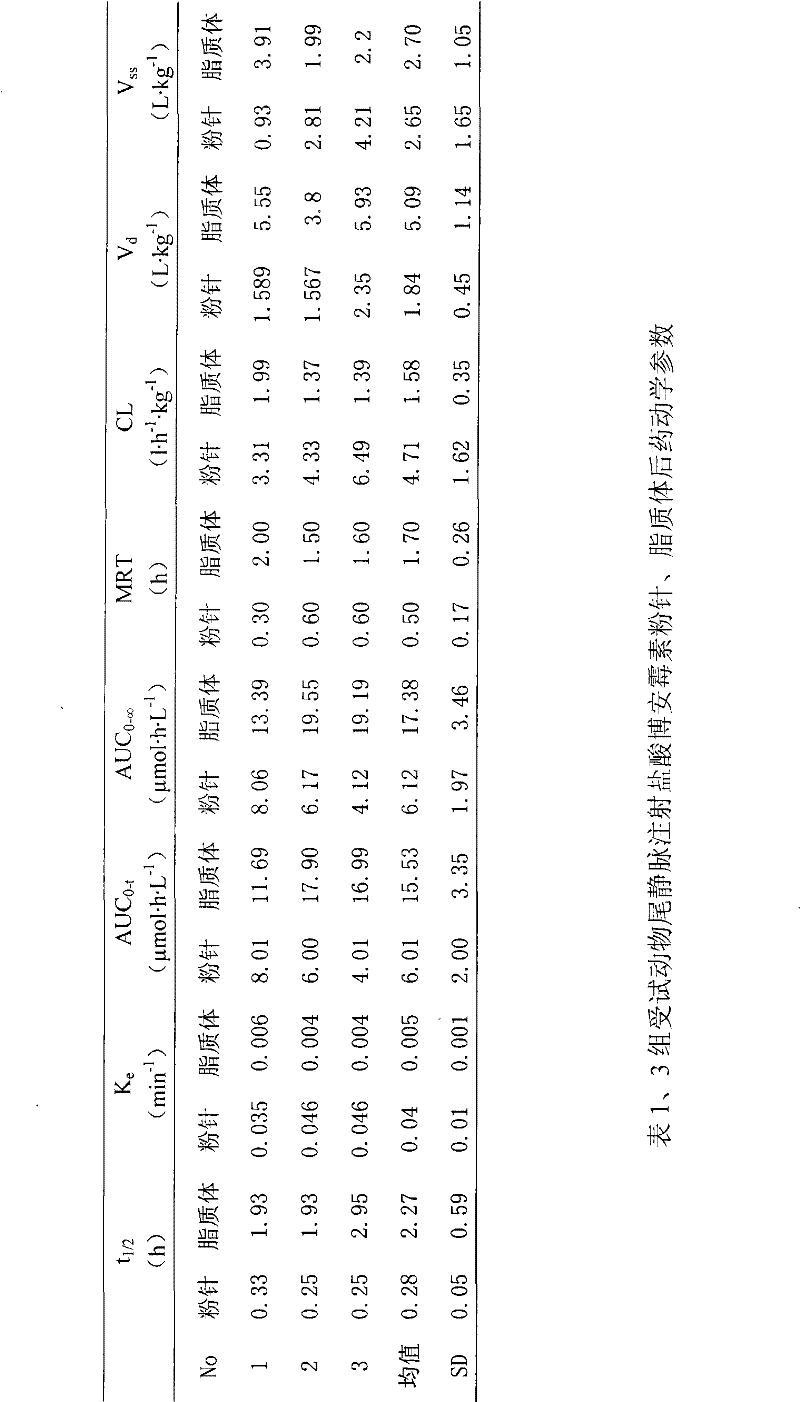
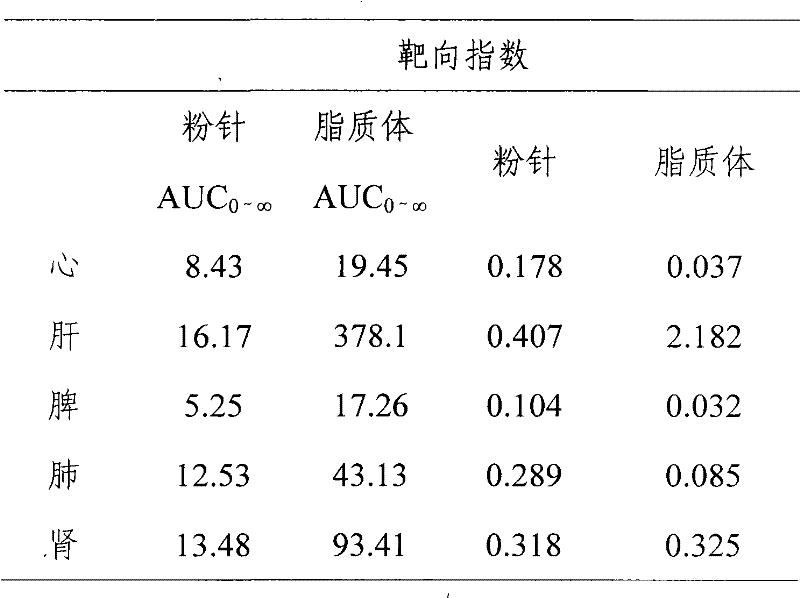
InactiveCN101513390AHigh encapsulation efficiencyMeet usage habitsSaccharide peptide ingredientsPharmaceutical non-active ingredientsSide effectFreeze-drying
The invention relates to the field of pharmaceutical preparation, in particular to hydrochloric acid boanmycin liposomes and a preparation method thereof. The hydrochloric acid boanmycin liposome comprises the hydrochloric acid boanmycin liposomes, phospholipids, cholesterin, citric acid, and sodium citrate, gradient regulator which respectively have the following mass percent: 0.5-3.0%, 3-30%, 1-10%, 0.5-3%, and 1-5%. The phospholipids is lecithin, double stearoyl phosphatidyl choline (DSPC), dipalmitoyl phosphatidyl choline (DPPC), dioleoyl phosphatidyl choline (DOPC) and the like. The invention is prepared by adopting the PH gradient method. Compared with the prior art, the hydrochloric acid boanmycin liposomes of the invention can reduce dosage, toxic side effect on human body and production cost and has the advantages such as targeting at some specific organs. The hydrochloric acid boanmycin liposomes of the invention can be prepared into parenteral solution or further prepared into freeze-dried powder hydrochloric acid boanmycin liposomes by adding a supporting agent.
Owner:SHENYANG PHARMA UNIVERSITY
Nelarabine parenteral solution and method for preparing same
The invention discloses nelarabine parenteral solution and a method for preparing the same. In the process for preparing the parenteral solution, temperature is kept at 50 to 90 DEG C at which the yield of the prepared nelarabine parenteral solution is greatly improved, which provides a good illustration for the influence of the temperature on the stability of the solution of basic medicament andpharmaceutical adjuvant in the process for preparing the nelarabine parenteral solution, and the nelarabine parenteral solution prepared at the temperature is of great scientific significance.
Owner:北京福瑞康正医药技术研究所
Transepithelial methods of using gamma aminobutyric acid compositions for pain relief
Disclosed are methods for alleviating, relieving, inhibiting, and eliminating acute or chronic pain in a patient suffering from such pain by contacting epithelial tissue of the patient with exogenous GABA (gamma-amino butyric acid).The epithelial tissue includes skin, nasal mucosa or membranes and other epithelial tissue such as buccal, palatal, sublingual, rectal, vaginal, thecal and the like. The GABA is supplied in compositions such as water, saline, buffered saline, parenteral solutions, lotions, semi-solids such as, gels, creams, pastes, salves, ointments, solids such as impregnated patches, impregnated rub-on solid compositions from which layers of GABA may be rubbed onto the skin, sublingual pills, suppositories and the like.
Owner:MOSKOWITZ MICHAEL H +2
Core bar component of injector and injector installing it
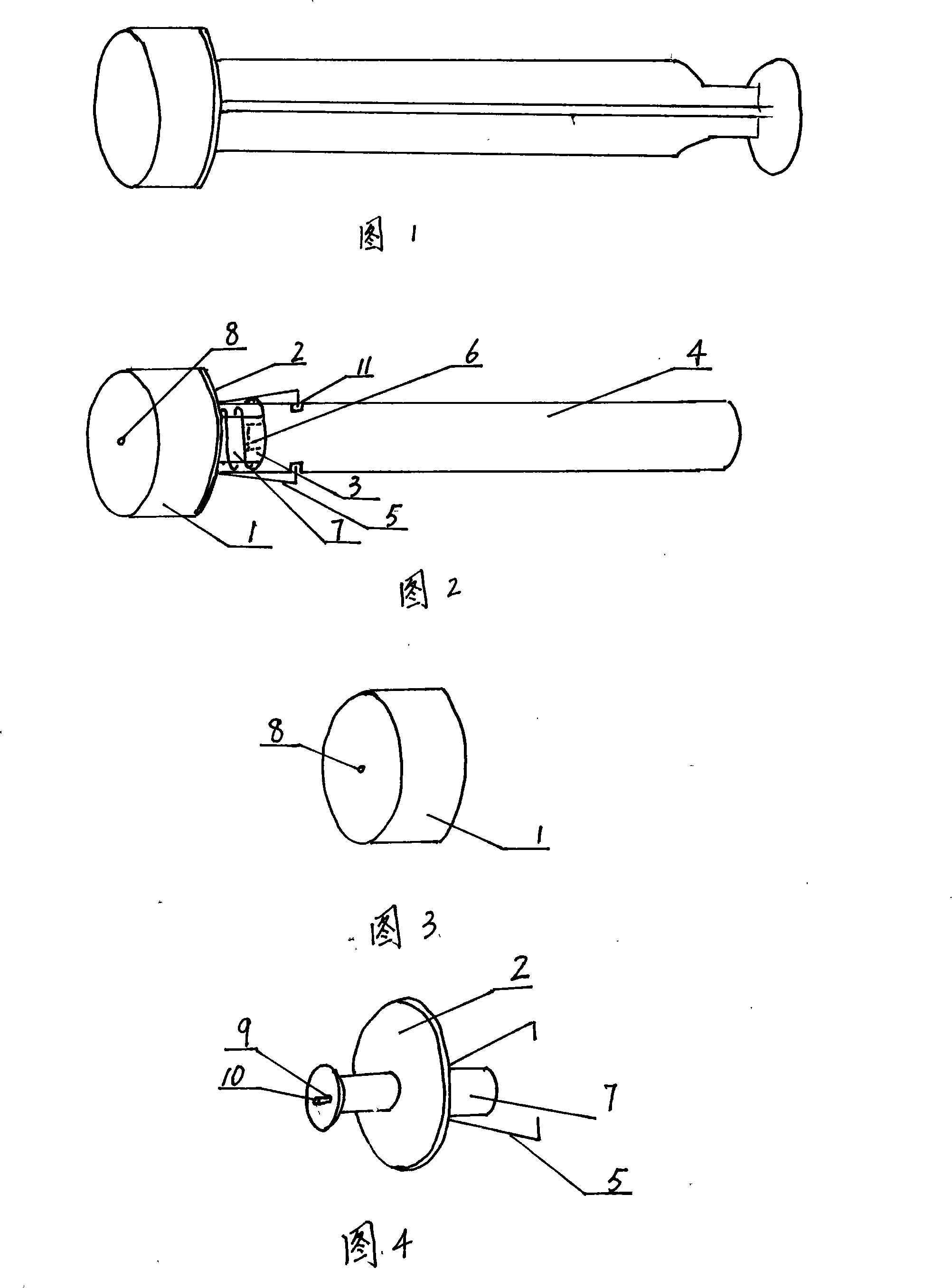
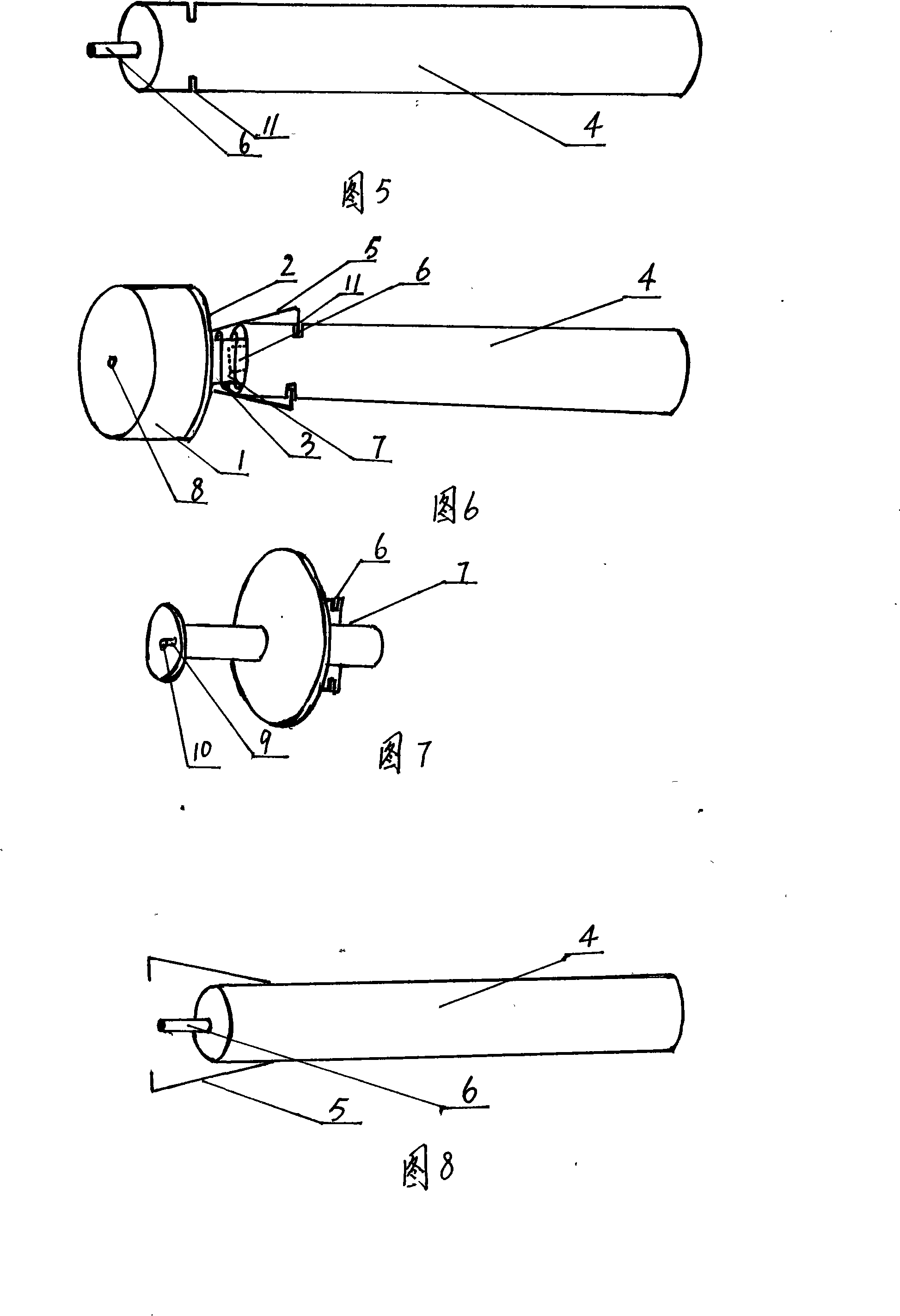
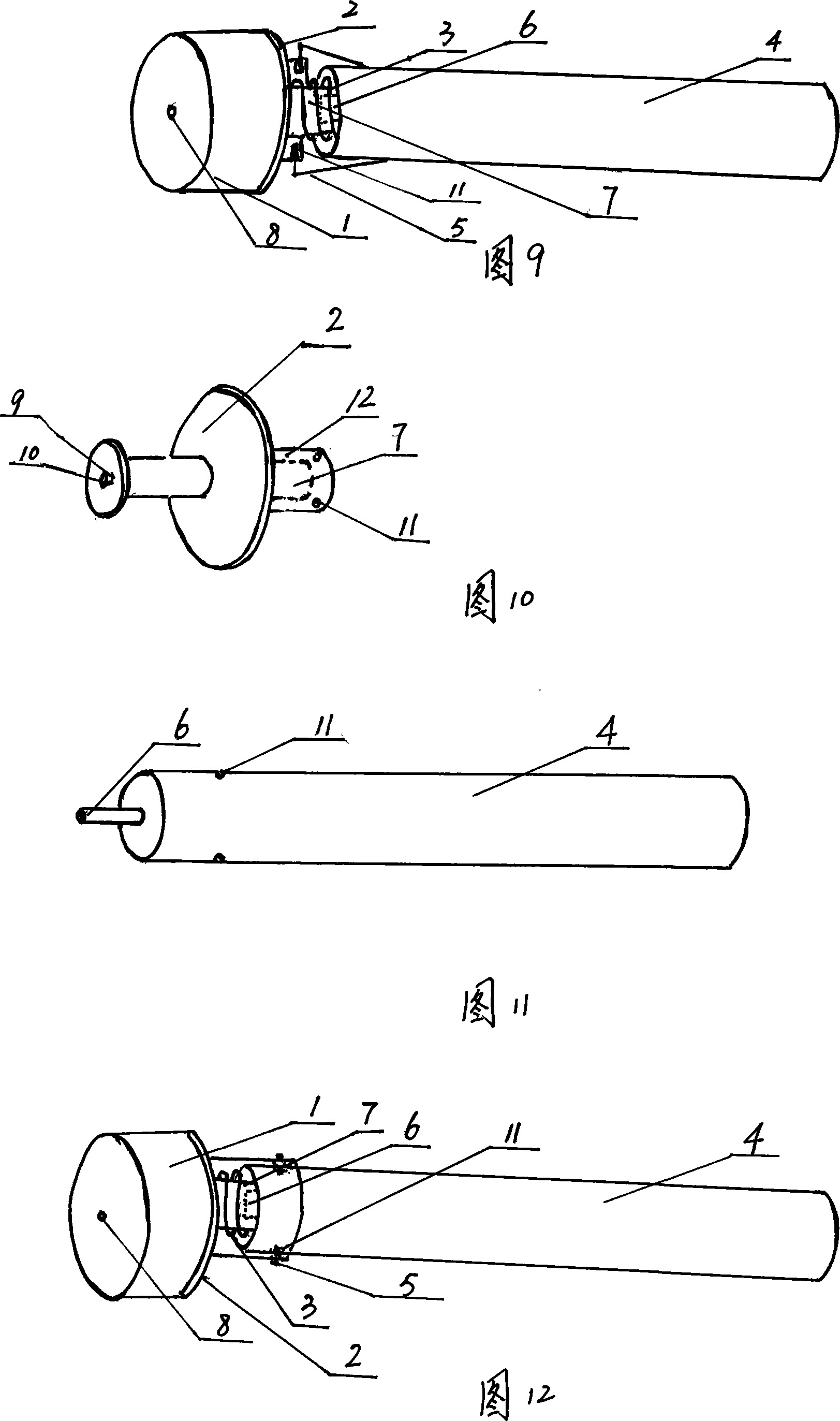
InactiveCN101204598AControlled inhalationControl injectionInfusion syringesIntravenous devicesAutomatic controlInjector nozzle
The invention relates to an injector core bar component and an injector employed by the component. The core bar component comprises a sealing plug, a sealing plug base, a spring, a push-pull rod (a tail end of the push-pull rod is arranged with a small sealing plug) and an elastic bayonet lock. Under an interaction of the spring and the elastic bayonet lock, the sealing plug base can be steadily combined with the push-pull rod through the compression and bounce of the spring, press and bounce of the elastic bayonet lock and insertion and extraction of the small sealing plug. An effective automatic control on absorption and effluence of parenteral solution can be realized. In the light of an injector nozzle plug (cover), the injector can not be used secondly. The invention not only can automatically control reusage of the injector, but also can provide safety and reliability, rational design, simple structure, convenient operation and low cost, and the invention can be widely popularized.
Owner:魏国
Parenteral solutions containing metolazone
Disclosed herein are parenteral solutions containing 7-halo-1,2,3,4-tetrahydro-3-aryl-6-quinazoline sulfonamide in N,N-dimethylactamide, polyethylene glycol and D5W useful in the treatment of hypertension, heart failure and renal disease leading to edematous states. Also disclosed are methods for preparing such solutions.
Owner:HYLORIS DEV SA
Medicament microcapsule and method for preparing injection thereof
InactiveCN101278923AQuality standardsImprove stabilityOrganic active ingredientsAntiviralsActive componentParenteral solutions
The invention relates to a medicinal microcapsule, taking the potassium sodium dehydroandroan drographolide succinate as medicinal active component, which is characterized in that the potassium sodium dehydroandroan drographolide succinate is coated by macromolecular polysaccharide membrane into the microcapsule. The invention also relates to a preparation method for potassium sodium dehydroandroan drographolide succinate injection; the potassium sodium dehydroandroan drographolide succinate is coated by the macromolecular polysaccharide membrane into the microcapsule and then made into the injection. The invention adopts the micro-capsule embedding technique to process the raw materials of the potassium sodium dehydroandroan drographolide succinate, thereby greatly improving the stability of the potassium sodium dehydroandroan drographolide succinate in aqueous solution, ensuring the mass stability of parenteral solution produced and being favorable for storing the product for a long time.
Owner:HAINAN LINGKANG PHARMA CO LTD
Parenteral formulation comprising siponimod
PendingCN111107837AHydroxy compound active ingredientsPharmaceutical delivery mechanismSiponimodParenteral solutions
The present invention relates to a pharmaceutical formulation of siponimod which can be administered parenterally. In particular, the present invention relates to a parenteral solution comprising siponimod and a method for preparing said solution.
Owner:NOVARTIS AG
Coenzyme Q10 injection
InactiveCN101278907BImprove securityImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismParenteral nutritionHydroxystearic Acid
The invention discloses the novel combined coenzyme Q10 parenteral solution, mainly containing the coenzyme Q10 as active component, polyethylene glycol 15-hydroxyl stearate as solubilizing agent and water for injection as solvent. One or more solvents of cosolvent, acidity regulator, osmoregulator and stabilizing agents can be also added. Low hemolyzation, extreme low histamine release and higher physiological tolerance of a novel solubilizing agent Solutol HS 15 adopted by the parenteral solution remarkably improves clinical medication security and the compliance of a patient; and the parenteral solution has the advantages of good stability, longer period of validity, higher medication convenience for a clinician, better storage and stable transportation, higher security for the clinical medication and better compliance of the patient. The parenteral solution also has the advantages of simple preparation technology, simple and convenient quality control and lower production cost, thereby being beneficial to industrialization production.
Owner:郑微
Hydrochloric acid boanmycin liposomes and preparation method thereof
InactiveCN101513390BHigh encapsulation efficiencyMeet usage habitsSaccharide peptide ingredientsPharmaceutical non-active ingredientsSide effectFreeze-drying
The invention relates to the field of pharmaceutical preparation, in particular to hydrochloric acid boanmycin liposomes and a preparation method thereof. The hydrochloric acid boanmycin liposome comprises the hydrochloric acid boanmycin liposomes, phospholipids, cholesterin, citric acid, and sodium citrate, gradient regulator which respectively have the following mass percent: 0.5-3.0%, 3-30%, 1-10%, 0.5-3%, and 1-5%. The phospholipids is lecithin, double stearoyl phosphatidyl choline (DSPC), dipalmitoyl phosphatidyl choline (DPPC), dioleoyl phosphatidyl choline (DOPC) and the like. The invention is prepared by adopting the PH gradient method. Compared with the prior art, the hydrochloric acid boanmycin liposomes of the invention can reduce dosage, toxic side effect on human body and production cost and has the advantages such as targeting at some specific organs. The hydrochloric acid boanmycin liposomes of the invention can be prepared into parenteral solution or further prepared into freeze-dried powder hydrochloric acid boanmycin liposomes by adding a supporting agent.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com