Composition containing glucosamine as well as preparation method and detection method thereof
A technology of glucosamine and glucosamine sulfate, which is applied in the field of preparation of glucosamine-containing compositions, can solve the problems of long freeze-drying time, toxic and side effects, and high energy consumption, and achieve the effects of reducing side effects, reliable application, and satisfying industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 Glucosamine sulfate injection (0.4g in terms of glucosamine sulfate)
[0051]
[0052] Preparation process: Take 80% of water to make 0.001mol / L hydrochloric acid solution, add glucosamine sulfate sodium chloride double salt and stir at 55°C to dissolve completely, then add 0.02% activated carbon, stir at 75°C for 20min, After decarbonization, add 0.01% activated carbon and lidocaine hydrochloride in the prescribed amount to the solution, stir at 45°C for 10 minutes, decarbonize, filter through 0.45 μm and 0.22 μm microporous membranes until clear, and the liquid is cooled Add water to a sufficient amount at 20°C, then adjust the pH value of the solution to 4.6, fill with 2ml, and finally sterilize at 121°C for 17 minutes.
[0053] Separately prepare a special solvent, as follows: 20g of diethanolamine plus 1000ml of water for injection to make 1000 vials. Preparation process: Weigh the above prescription amount of alkaline regulator diethanolamine, add...
Embodiment 2
[0054] Embodiment 2 Glucosamine sulfate injection (0.4g in terms of glucosamine sulfate)
[0055]
[0056] Preparation process: Take 80% water to prepare 0.001mol / L hydrochloric acid solution, add glucosamine sulfate sodium chloride double salt and stir to dissolve completely, then add 0.02% activated carbon, stir at room temperature for 20min, decarbonize, and then pour into the solution Add 0.01% activated carbon and lidocaine hydrochloride in the prescription amount, stir at room temperature for 10 minutes, decarbonize, filter through 0.45 μm, 0.22 μm microporous membranes until clarification, add water to a sufficient amount when the liquid is cooled to 20°C , and then adjust the pH value of the solution to 4.6, fill with 2ml, and finally sterilize at 121°C for 17min.
[0057] Special solvent is prepared with the method of embodiment 1.
experiment example 1
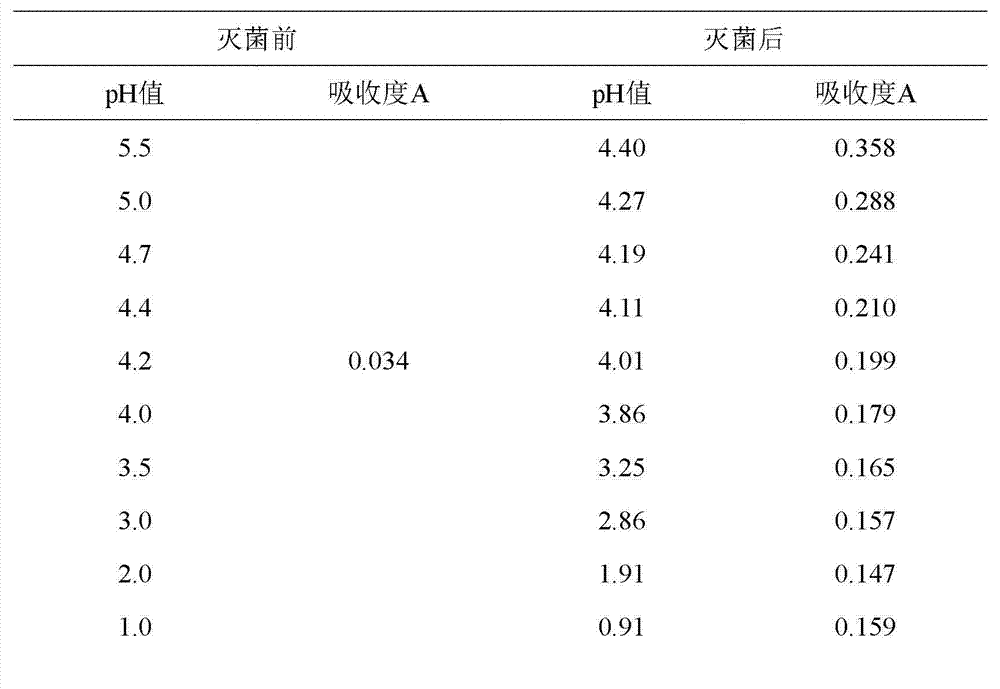
[0058] Experimental example 1: Due to the reaction process of 5-hydroxymethylfurfural, it is considered that this process is composed of a series of elementary reactions such as protonation, dehydration and deprotonation, among which protonation is relatively easy, and the energy barrier of deprotonation reaction is relatively low. High, is the rate-determining step of the conversion process. Therefore, changes in temperature have a greater effect on the decomposition of glucosamine than changes in pH. According to the preparation method of Example 4 in the CN201010270516 patent application, take 80% water to prepare hydrochloric acid solution, after the active ingredient is dissolved and add enough water, the pH reaches 5.5, 5.0, 4.7, 4.4, 4.2, 4.0, 3.5, 3.0 , 2.0, 1.0, and finally sterilized at 121°C for 17min. Simultaneously, the pH of Comparative Example 1 reached 5.5, 5.0, 4.7, 4.6, 4.4, 4.2, 4.0, and 3.5 before sterilization, and finally sterilized at 121° C. for 17 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com