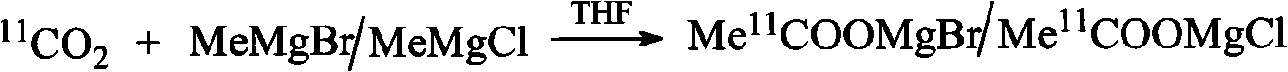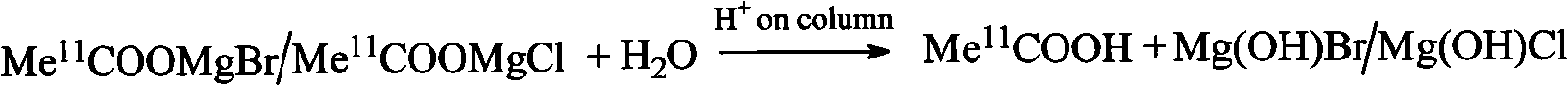C-acetate preparation method
A technology of acetate and acetyl bromide, which is applied in the field of 11C-acetate preparation, can solve the problems of easy blockage, synthesis and failure of pipelines, and achieve the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1.1 11 Synthesis of C-CA
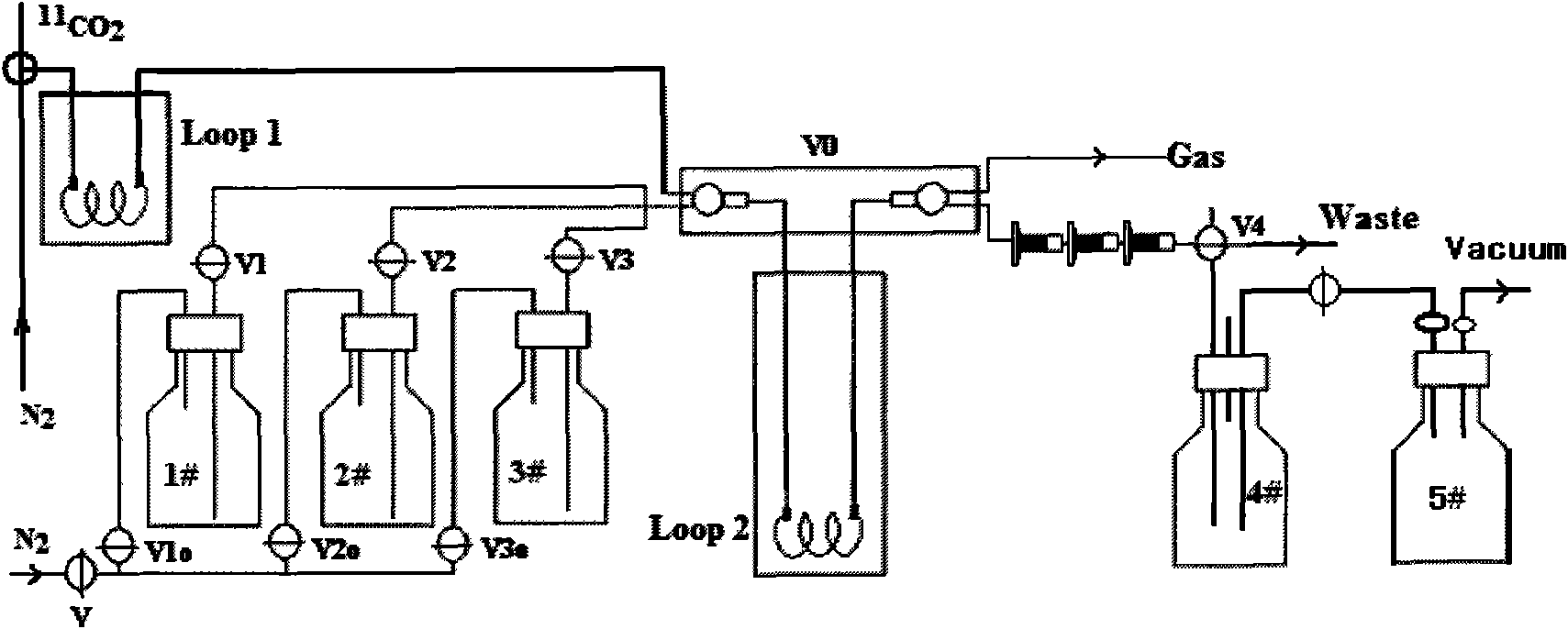
[0030] Synthesized using a slightly modified carbon-11 choline / methionine synthesis module 11 In addition to the change of the purification column and reagents, C-AC only needs a slight change in the structure. Only 5# bottles and a vacuum pump are added to the original module to achieve 11 C-AC is fully automatically synthesized, and its synthesis process is shown in figure 1 .
[0031] Before synthesis, take 0.1 mL of anhydrous tetrahydrofuran solution of methylmagnesium bromide with a concentration of 0.87-1.5 mol / L and put it in Loop2. by PETtrace Cyclotron via 14 N(p,α) 11 C nuclear reaction production 11 CO 2 , 11 CO 2Trapped in Loop1 ring under liquid nitrogen cooling. At room temperature, with 10mL / min nitrogen as the carrier gas, 11 CO 2 In the LOOP2 ring, it reacts with methylmagnesium bromide to generate the intermediate acetylmagnesium bromide adduct, and the solvent tetrahydrofuran is passed to the waste gas bottle und...
Embodiment 2
[0038] Adopt 0.2mL 1.0mol / L methylmagnesium chloride to replace the 0.1mL 1.5mol / L methylmagnesium bromide in embodiment 1, radioactive decline in waste liquid, in crude product 11 CO 2 As the content increased, the labeling rate decreased slightly.
[0039] The present invention uses an integrated small column to complete the hydrolysis and purification work in the column. Compared with the small column purification method reported in the existing literature (references 1, 3-7), this method is very simple and easy to implement 11 The automatic synthesis of C-AC avoids production failure caused by the use of Ag+ column to generate silver halide precipitation to block the pipeline, and the production cost is significantly reduced. intermediate 11 C-acetyl bromide (or chloride) magnesium adduct can be hydrolyzed in the SEP-PAK TSCX column; the impurities contained in the product are mainly 11 CO 2 , 11 C-acetone and 11 C-carbonate, 11 C-tert-butanol, tetrahydrofuran, Mg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com