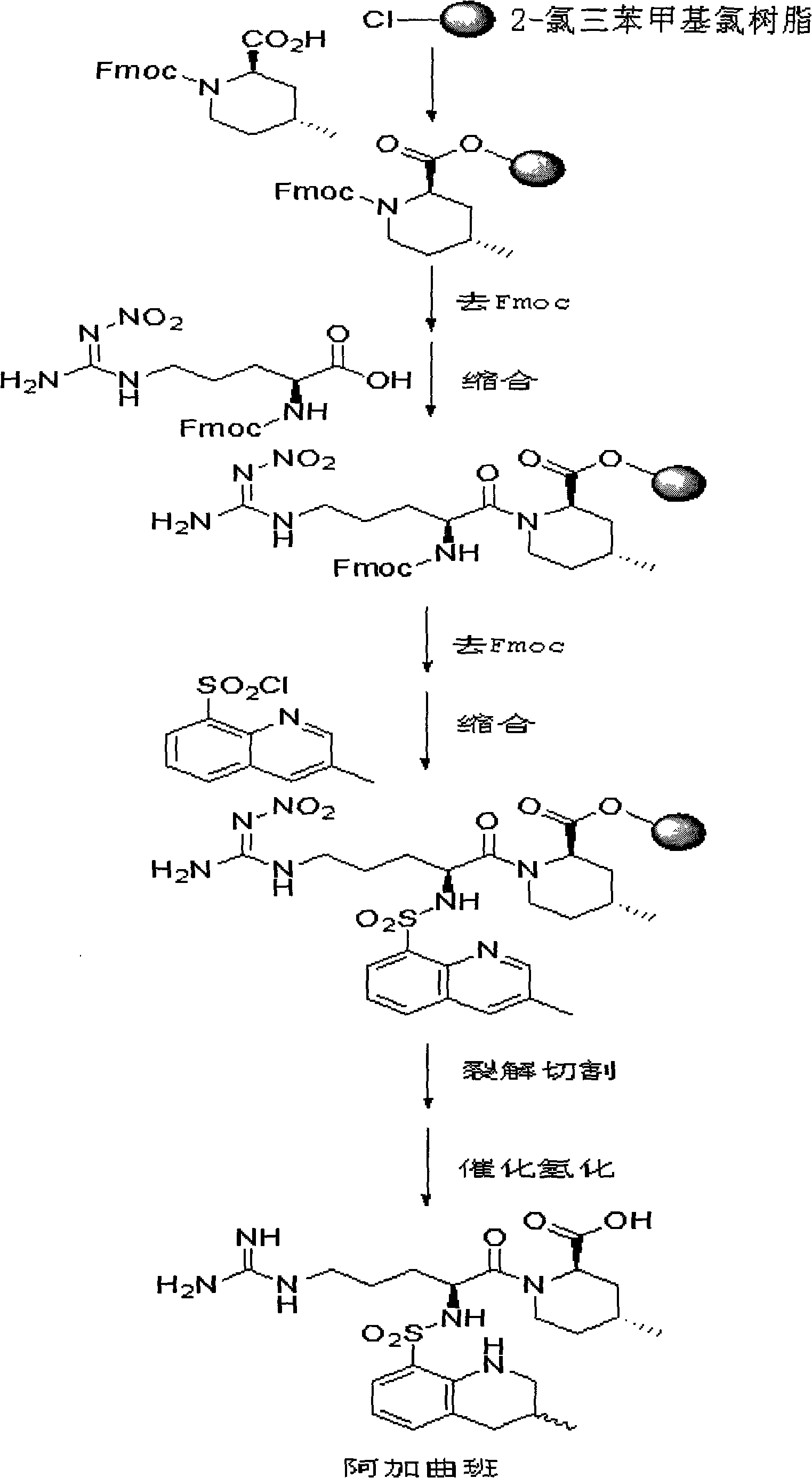
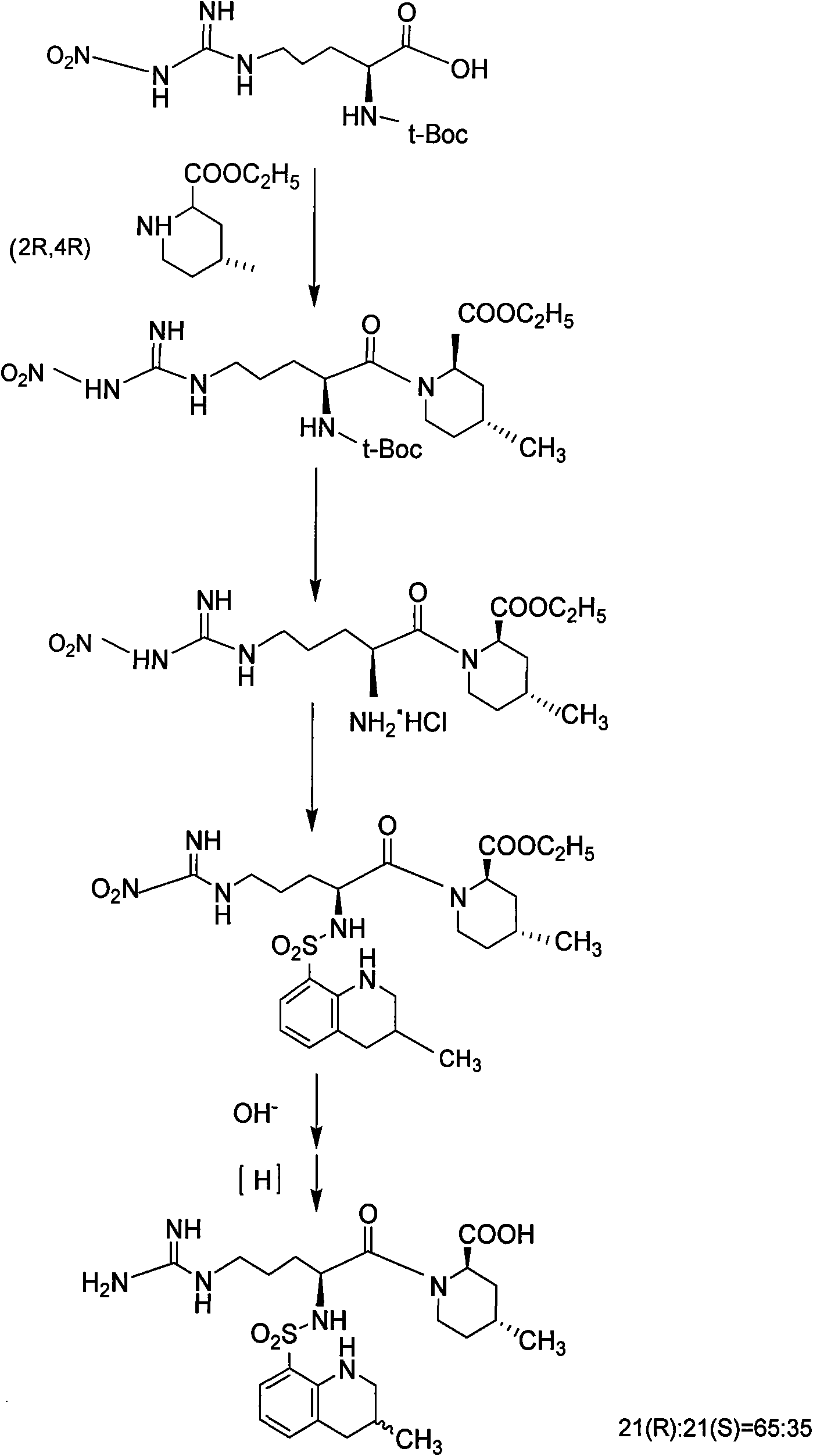
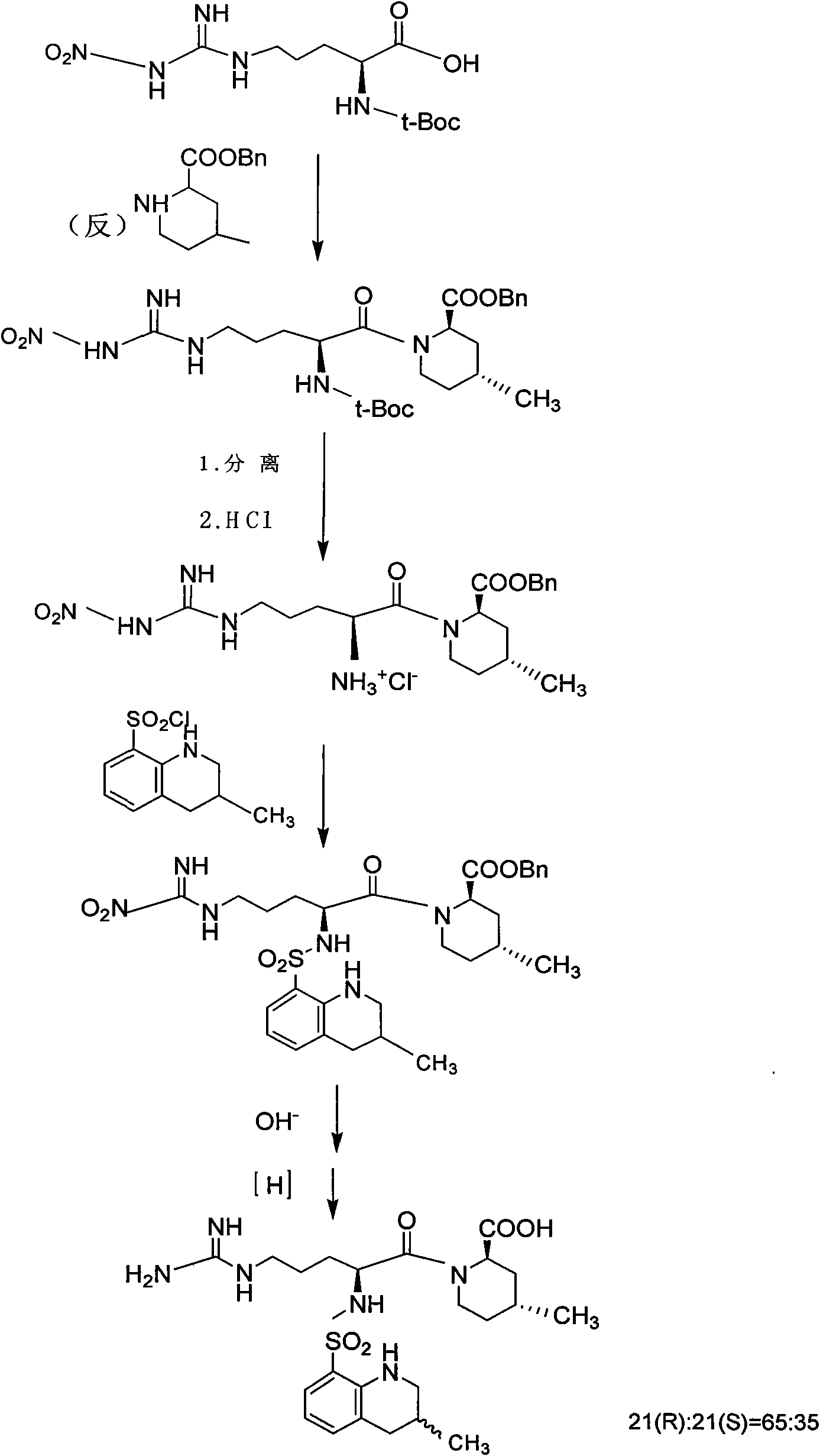
New method for synthesizing argatroban by combining solid phase method and liquid phase method
A technology of argatroban and methyl, which is applied in the field of synthesis of polypeptide compounds, can solve the problems of difficult purification and separation of intermediates and target products, low yield of argatroban, etc., and achieve considerable economic and practical value. Ease of handling and simple operation of the response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid-2-chlorochloro resin
[0043] Add 200.0 g of 2-chlorochloro resin (substitution degree: 0.5 mmol / g) into a 1000 ml round bottom flask, add DMF 600 ml, and stir vigorously. In an ice-water bath, 36.5g (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid and equal molar ratios of DIC, HOBt and DIPEA were added to the above-mentioned round-bottomed flask filled with resin and reacted for 2 hours . After the reaction is completed, filter, wash 3 times with DMF, and wash 3 times with DCM, then shrink with methanol for 30 minutes to obtain (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid-2-chlorochloro resin, and detect The degree of substitution is 0.48mmol / g, and the yield is 96%.
Embodiment 2
[0044] Example 2: Preparation of (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid-2-chlorochloro resin
[0045] Add 450.0 g of 2-chlorochloro resin (substitution degree: 0.8 mmol / g) into a 2000 ml round bottom flask, add DMF1000 ml and stir vigorously. In an ice-water bath, 262.8g (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid and equal molar ratios of DIC, HOBt and DIPEA were added to the above-mentioned round-bottomed flask with resin and reacted for 2 hours . After the reaction is completed, filter, wash 3 times with DMF, and wash 3 times with DCM, then shrink with methanol for 30 minutes to obtain (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid-2-chlorochloro resin, and detect The degree of substitution is 0.72mmol / g, and the yield is 90%.
Embodiment 3
[0046] Embodiment 3: Preparation of (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid-2-chlorochloro resin
[0047] Add 300.0 g of 2-chlorochloro resin (substitution degree: 0.3 mmol / g) into a 2000 ml round bottom flask, add 800 ml of DMF and stir vigorously. In an ice-water bath, 65.7g (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid and equal molar ratios of DIC, HOBt and DIPEA were added to the above-mentioned round-bottomed flask filled with resin and reacted for 2 hours . After the reaction is completed, filter, wash 3 times with DMF, and wash 3 times with DCM, then shrink with methanol for 30 minutes to obtain (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid-2-chlorochloro resin, and detect The degree of substitution is 0.29mmol / g, and the yield is 97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com