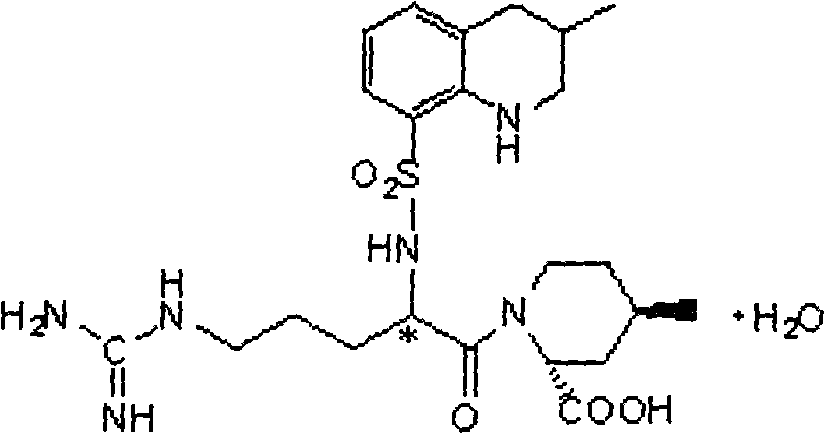
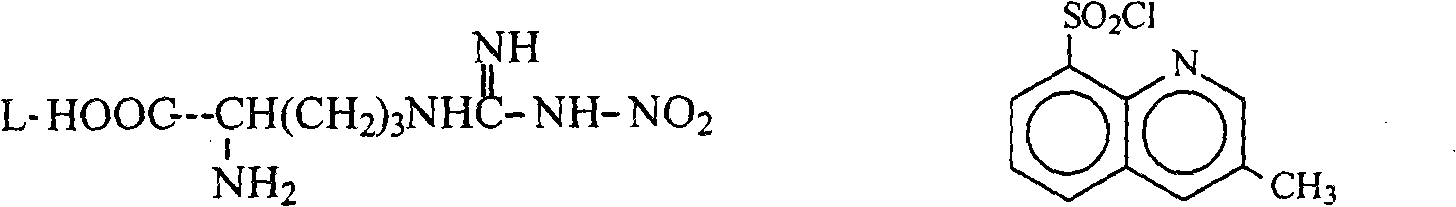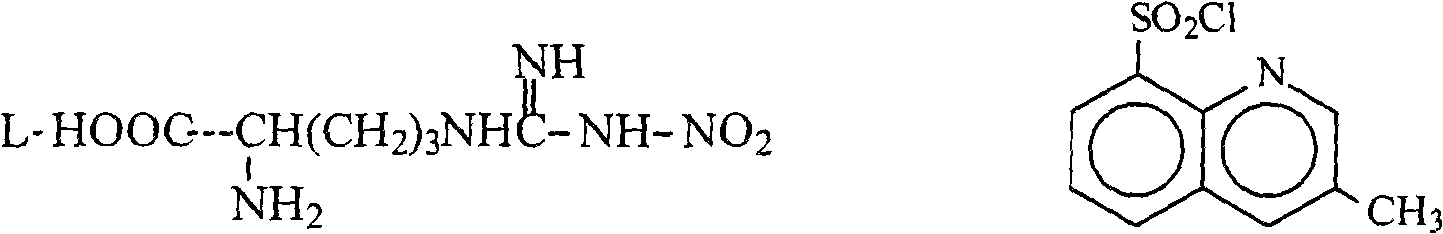Synthetic method of argatroban and intermediate thereof
A technology of argatroban and intermediates, applied in the field of drug synthesis, which can solve the problems of difficult removal of water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 27g of anhydrous sodium carbonate to the reaction flask, add water to dissolve it, add 47g N G -Nitro-L-arginine 0.214mol), stirred and heated to about 60°C to dissolve, cooled to 25°C, added dropwise 50g (0.206mol) of tetrahydrofuran solution of 3-methyl-8-quinolinesulfonyl chloride, 15 Complete the addition in 1 minute, react at room temperature for 5 hours, evaporate the tetrahydrofuran after the reaction is completed, wash the cooled water phase with 100ml×2 chloroform, add 300ml tetrahydrofuran to the water phase, adjust the pH to about 2.5 with 10% hydrochloric acid, add sodium chloride solid saturated water layer, the tetrahydrofuran layer was separated, and the aqueous layer was extracted and combined with tetrahydrofuran (400ml×3), dried over anhydrous sodium sulfate, overnight. The desiccant was removed by filtration, and 120 g of 4A molecular sieves were added to the filtrate to dry overnight, and then placed to be tested and used.
[0030] Intermediate ...
Embodiment 2
[0033] Add 1.62kg of anhydrous sodium carbonate to a 300L reaction kettle, add water and stir to dissolve, add N G -Nitro-L-arginine 2.72kg (12.4mol), stirred and heated to about 60°C, the solution was clear. Cool down to 25°C, inhale 3.0kg (12.4mol) of tetrahydrofuran solution of 3-methyl-8-quinolinesulfonyl chloride, and 31.8kg of tetrahydrofuran into a 20L dropping bottle, slowly drop into the reaction kettle, and complete the addition in about 40 minutes . The temperature was controlled at 25°C for 5 hours. After the reaction was completed, tetrahydrofuran was distilled off under reduced pressure (control internal temperature < 50°C). Cool the reaction solution below 30°C, wash it with chloroform 6L×2, then add 16kg tetrahydrofuran to the water, adjust the pH to about 2.5 with 10% hydrochloric acid while stirring, add 7.5kg sodium chloride and stir evenly, and let it stand for stratification. The tetrahydrofuran layer was separated, and the water layer was extracted wit...
Embodiment 3
[0036]Add 1.62kg of anhydrous sodium carbonate to a 300L reaction kettle, add water and stir to dissolve, add N G -Nitro-L-arginine 3.5kg (15.9mol), stirred and heated to about 60°C, the solution was clear. Cool down to 25°C, inhale 3.6kg of a tetrahydrofuran solution of 3-methyl-8-quinolinesulfonyl chloride (14.9mol), and 98kg of tetrahydrofuran into a 20L dropping bottle, slowly drop them into the reaction kettle, and complete the addition in about 40 minutes. The temperature was controlled at 25°C for 5 hours. After the reaction was completed, tetrahydrofuran was distilled off under reduced pressure (control internal temperature < 50°C). Cool the reaction solution below 30°C, wash it with 12L×2 chloroform, then add 32kg tetrahydrofuran to the water, adjust the pH to about 2.5 with 10% hydrochloric acid while stirring, add 10kg ammonium chloride and stir evenly, let stand to separate layers, and separate The tetrahydrofuran layer was removed, and the aqueous layer was extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com