Argatroban byproduct and its synthesis, separation and identification method
A technology for argatroban and by-products, which is applied in the fields of argatroban by-products and synthesis and separation and identification, can solve the problems of difficult separation, difficult purification, and difficulty in reaching the standard of impurity content of product Argatroban, and achieves mild conditions and steps. Reasonable design and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
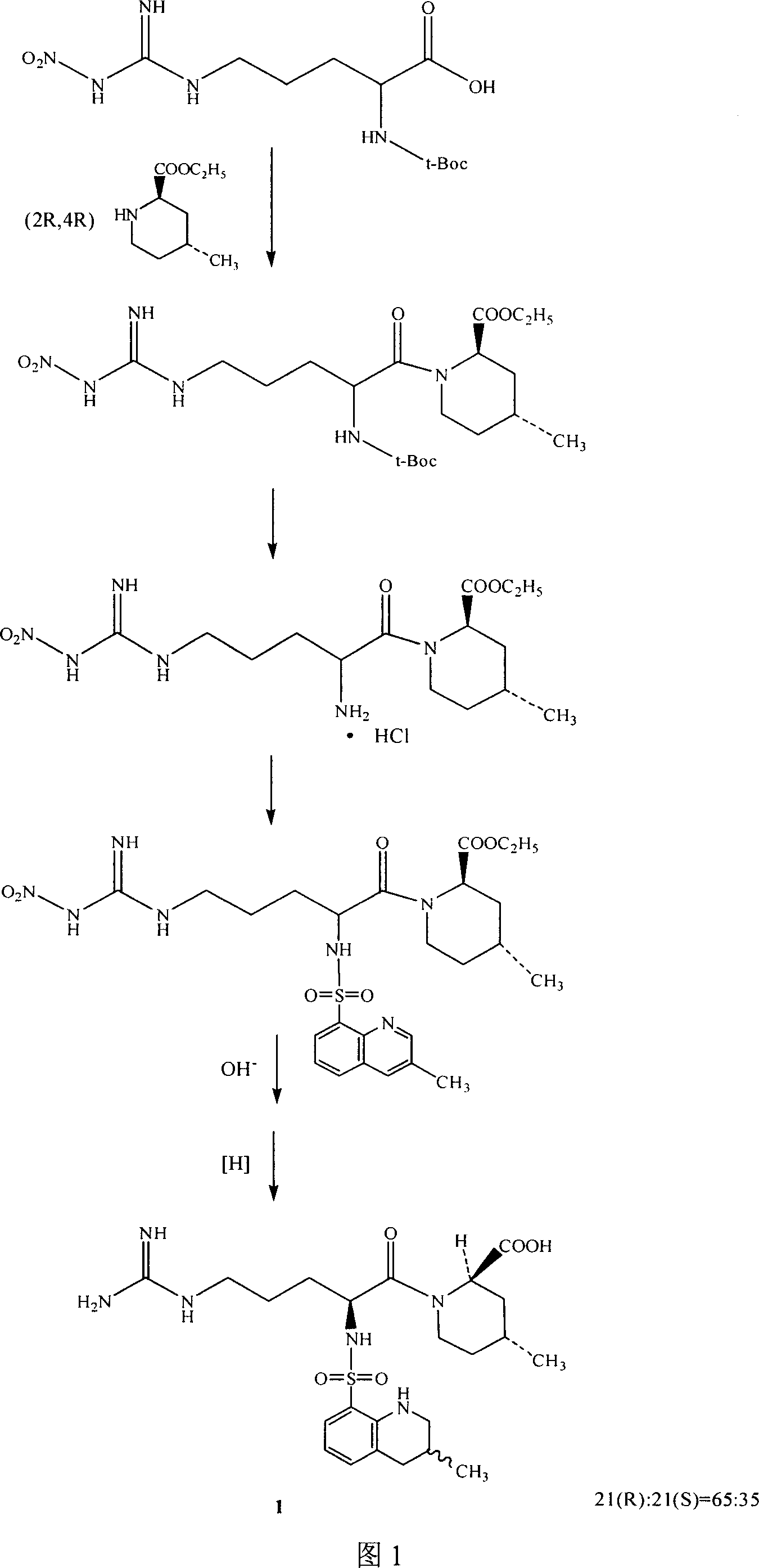
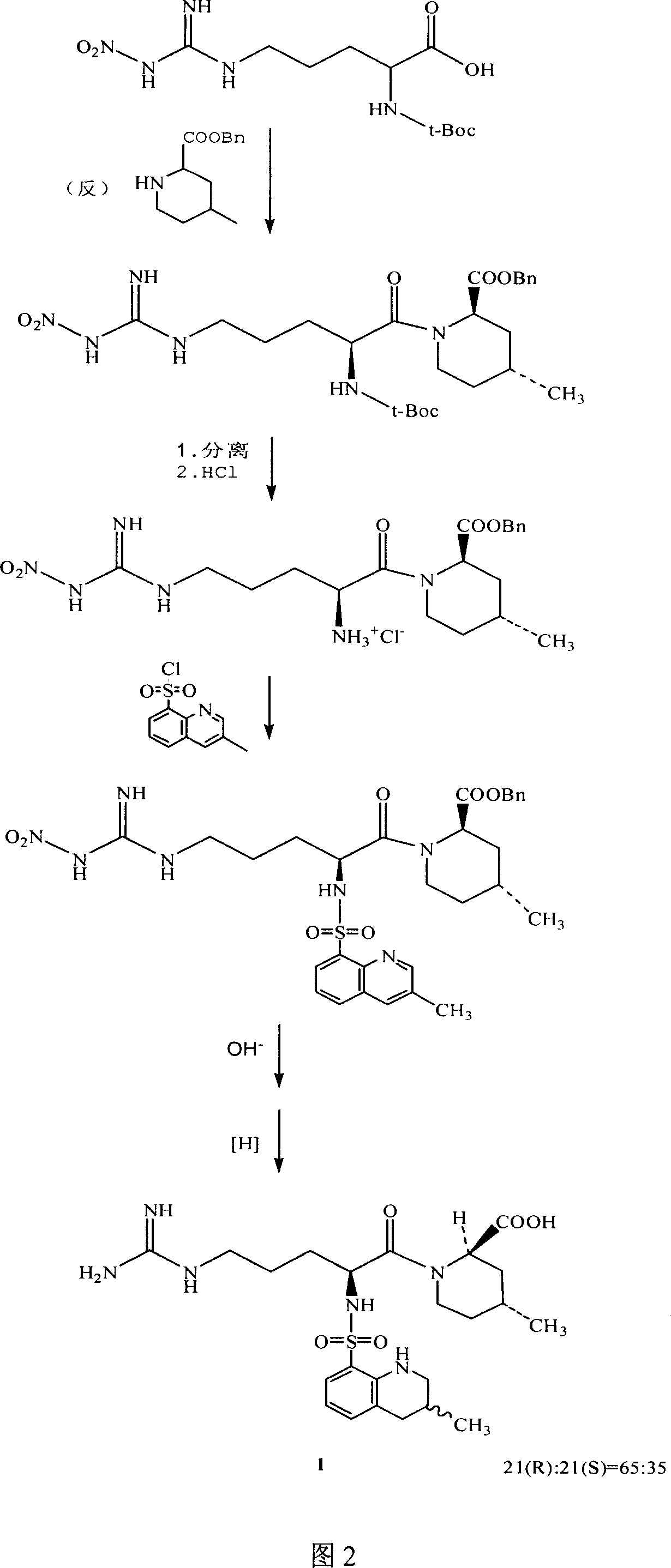
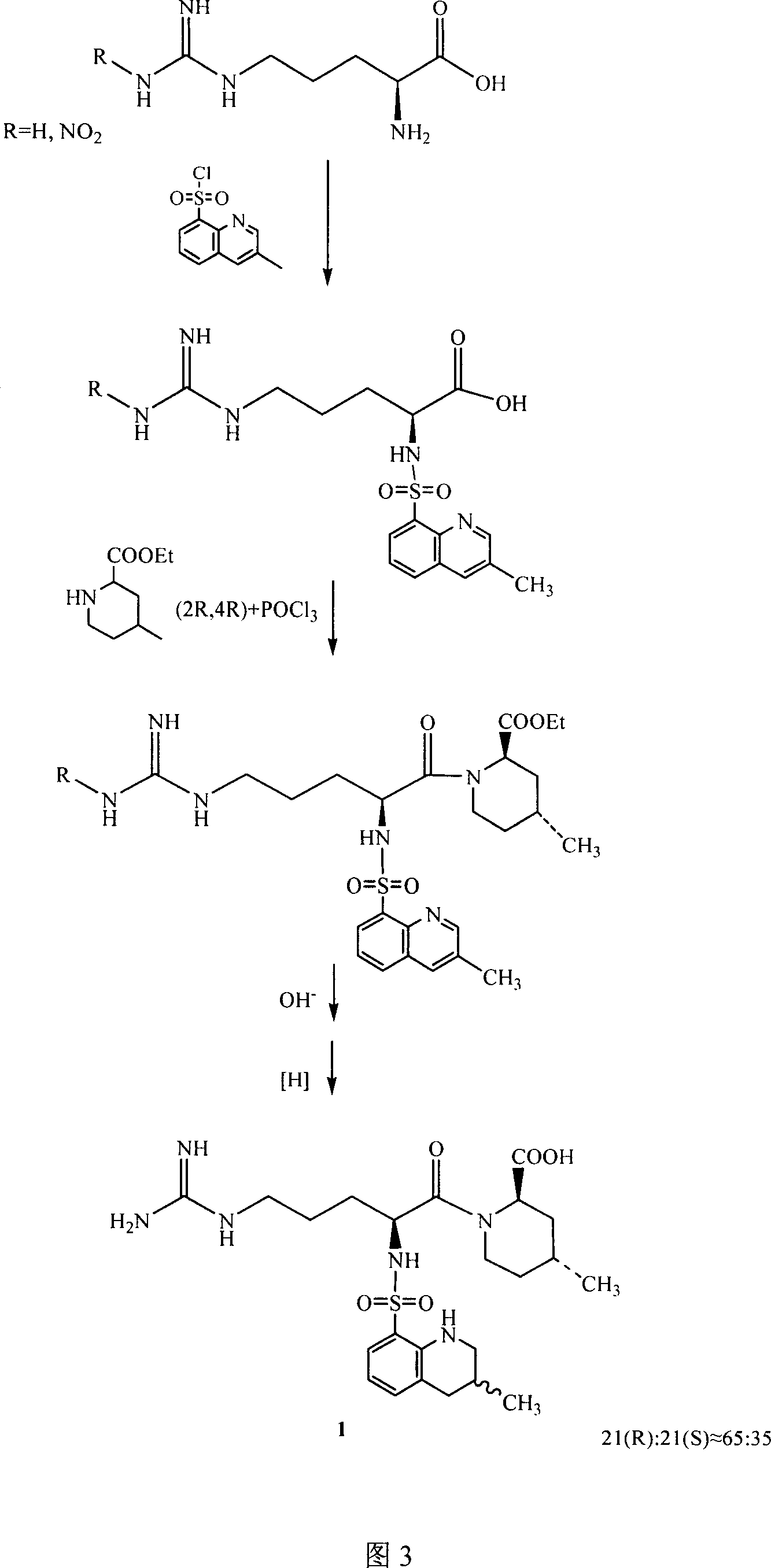
[0033] See (US 4 258 192) for the preparation example of intermediate 2 (compound 2), and see accompanying drawing 1 for the reaction formula.
example 2
[0034] Example 2, palladium carbon catalytic hydrogenation method:
[0035] 1 g of compound 2 prepared in Example 1, 0.02 g of triphenylphosphine, 0.2 g of 5% palladium on carbon, 13 ml of absolute ethanol and 4 ml of glacial acetic acid were successively added into a 100 ml autoclave. Control the external temperature of the autoclave at 65-70°C, feed hydrogen gas, keep the pressure at 10 MPa, follow the reaction by HPLC (high performance liquid chromatography), react for 24 hours, and stop the reaction. Remove palladium carbon by filtration, desolventize, dissolve with 20ml of dichloromethane, adjust pH=7 with saturated sodium bicarbonate. The organic layer was separated, washed with 10 ml of saturated sodium chloride, left to stand, the organic layer was separated, dried over anhydrous magnesium sulfate, filtered, and dichloromethane was removed to obtain 0.75 g of a white solid, which was compound 3, with a yield of 82%. .
[0036] Compound 3 structure identification:
[0...
example 3
[0052] Example 3. stannous chloride method:
[0053] Add 1g of compound 2 prepared in Example 1, 2.4g of stannous chloride and 40ml of absolute ethanol into a 100ml four-neck flask, stir and react at 20-25°C for 24 hours, follow the reaction by HPLC, the content of compound 2 no longer decreases, stop the reaction. Ethanol was removed in vacuo, water and dichloromethane were added, the aqueous layer was extracted with dichloromethane, the organic layers were combined, washed with water, washed with saturated sodium chloride, washed with water, dried over anhydrous magnesium sulfate, filtered, and precipitated to obtain 0.6 g of compound 3 . Yield 65%. HPLC retention time was 8.5 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com