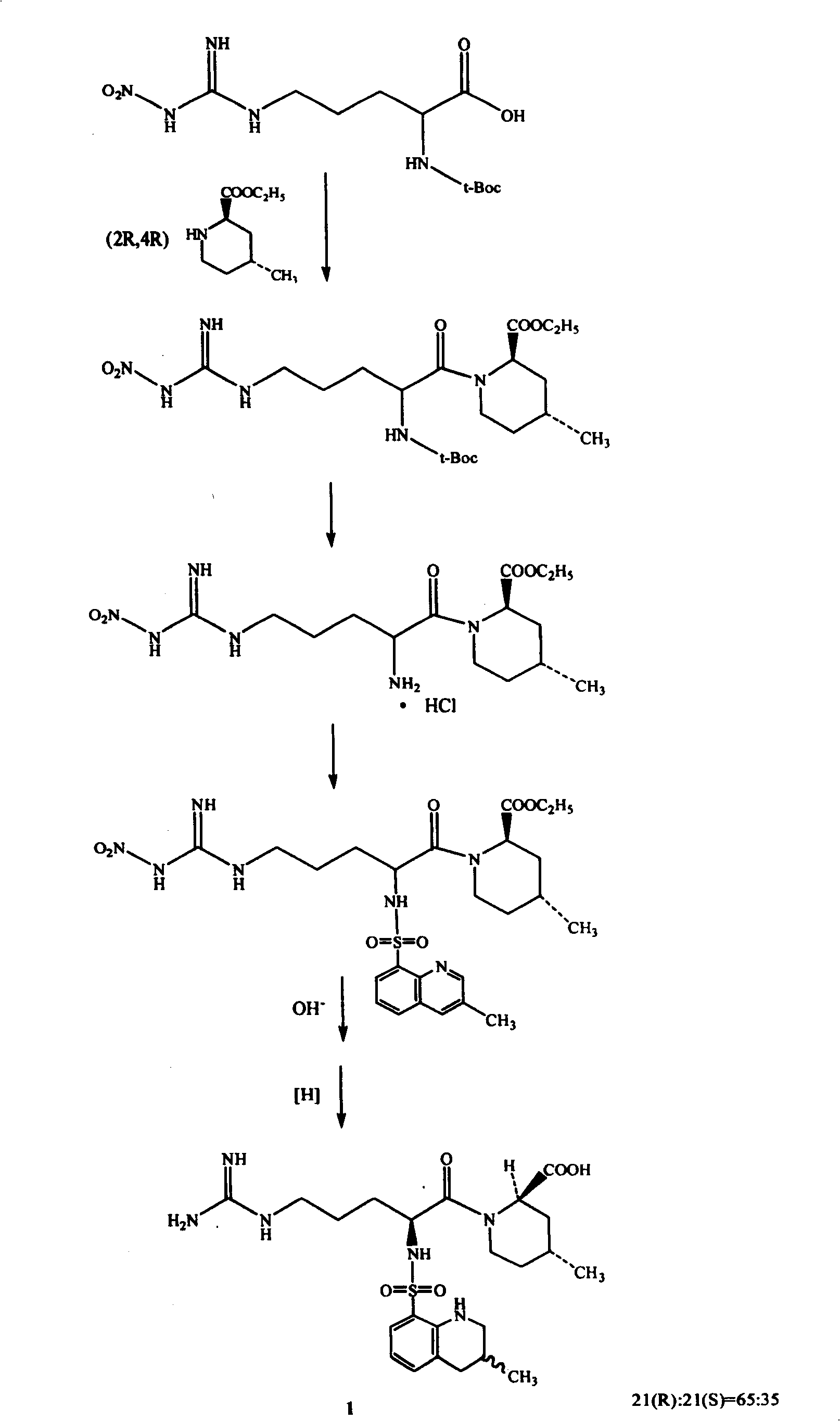
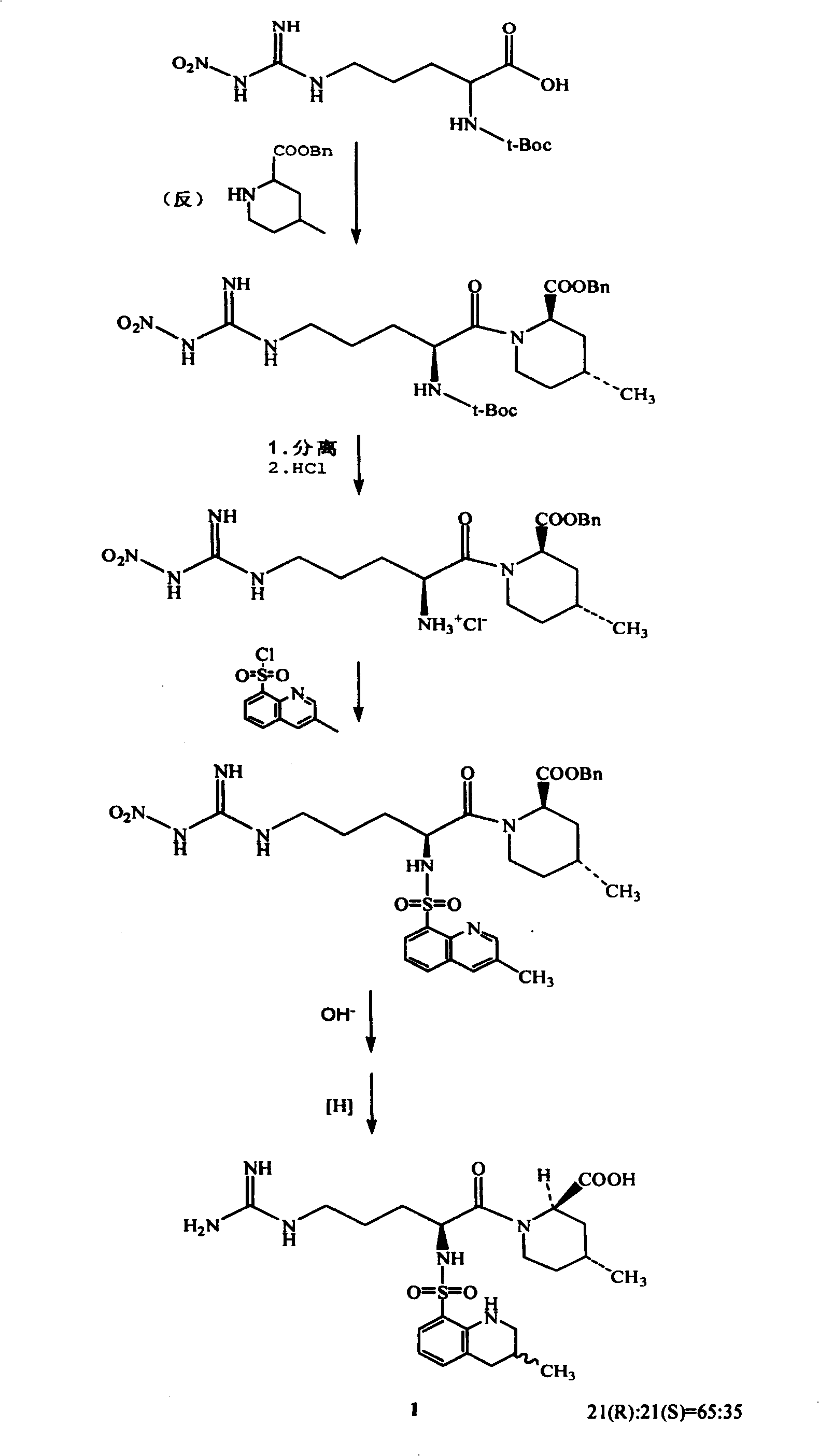
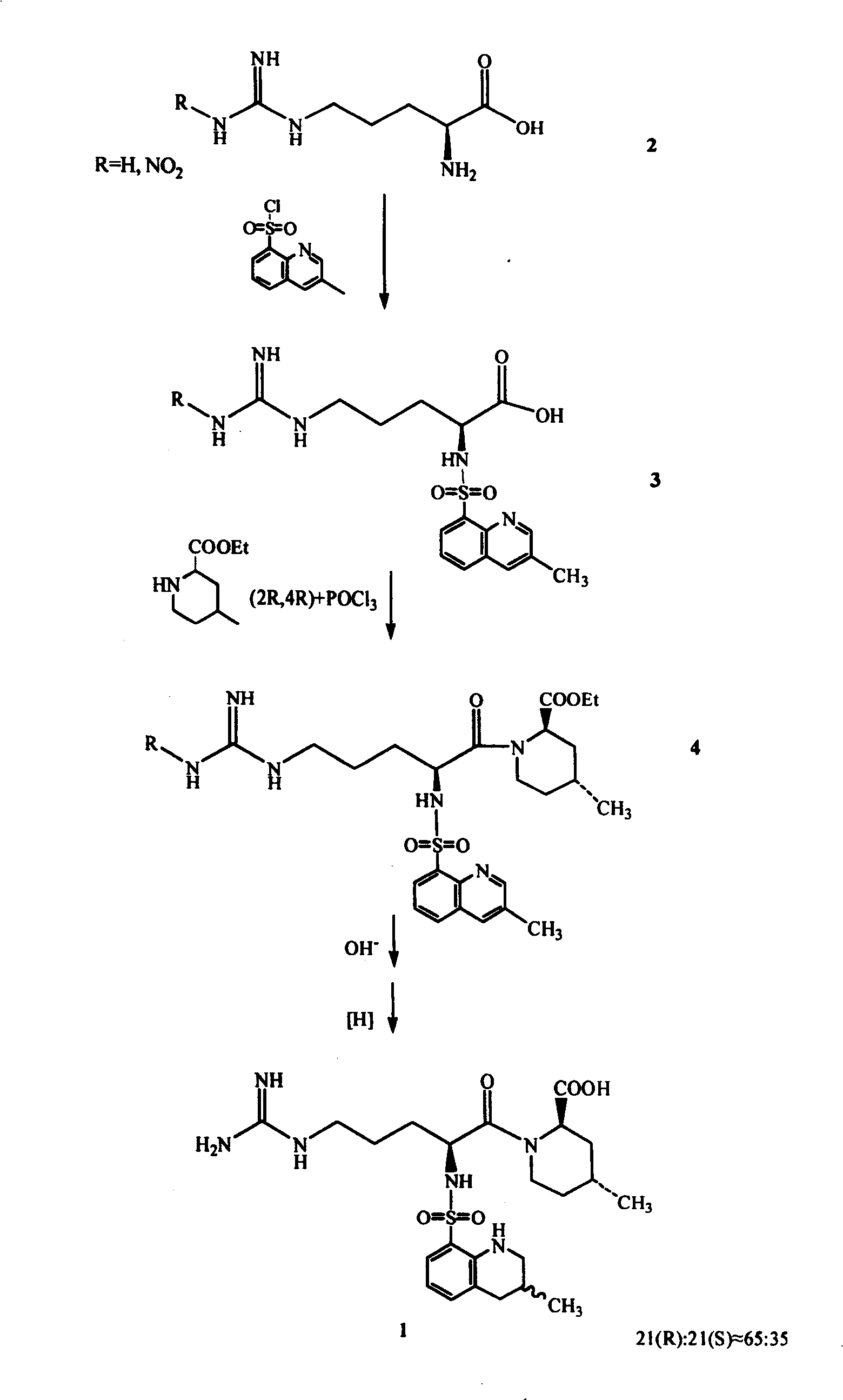
Process for preparing argatroban hydrate
A technology of argatroban and hydrate, which is applied in the field of preparation of argatroban hydrate, can solve problems such as nonconformity, low yield, and large ratio change, and achieve mild operating conditions, high yield, and high product quality. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1. 65g Argatroban (R: S=65: 35) solid is added in 4200ml distilled water, slowly warming up to reflux, after all the solid dissolves, naturally cool to room temperature, white crystals are separated out, filter. Wash twice with water and dry within 100°C to obtain Argatroban monohydrate Add 65g of Argatroban (R:S=65:35) to 4200ml of distilled water, slowly heat up to reflux, after all the solids are dissolved, naturally cool to room temperature, and white Crystallized, filtered. Wash twice with water and dry within 100°C to obtain 59 g of Argatroban monohydrate. Yield 87.3%. 21(R):21(S)=64.5:34.5 (HPLC method), water content 3.55% (Karl Fischer method).
[0022] Elemental analysis:
[0023] Experimental value: S: 5.95% N: 15.05% H: 7.35%
[0024] Calculated (C23H38N6O6S): S: 6.08% N: 15.96% H: 7.27%
Embodiment 2
[0025] Example 2. 50 grams of Argatroban (R:S=65:35) was added to 4000ml of distilled water, the temperature was raised to 90° C., the reaction was stirred for two hours, then naturally cooled to room temperature, white crystals were precipitated, and filtered. Wash twice with water and dry within 100°C to obtain 46.8 g of Argatroban monohydrate with a yield of 90.4%. 21(R):21(S)=64.8:35.2 (HPLC method), water content 3.34% (Karl Fischer method).
[0026] Elemental analysis:
[0027] Experimental value: S: 5.87% N: 15.23% H: 7.05%
[0028] Calculated (C23H38N6O6S): S: 6.08% N: 15.96% H: 7.27%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com