Novel crystal form of argatroban and preparation method thereof
A technology of argatroban and crystal form, applied in the field of pharmaceutical crystallization, can solve the problems of harming methanol, consuming large amounts of water and energy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
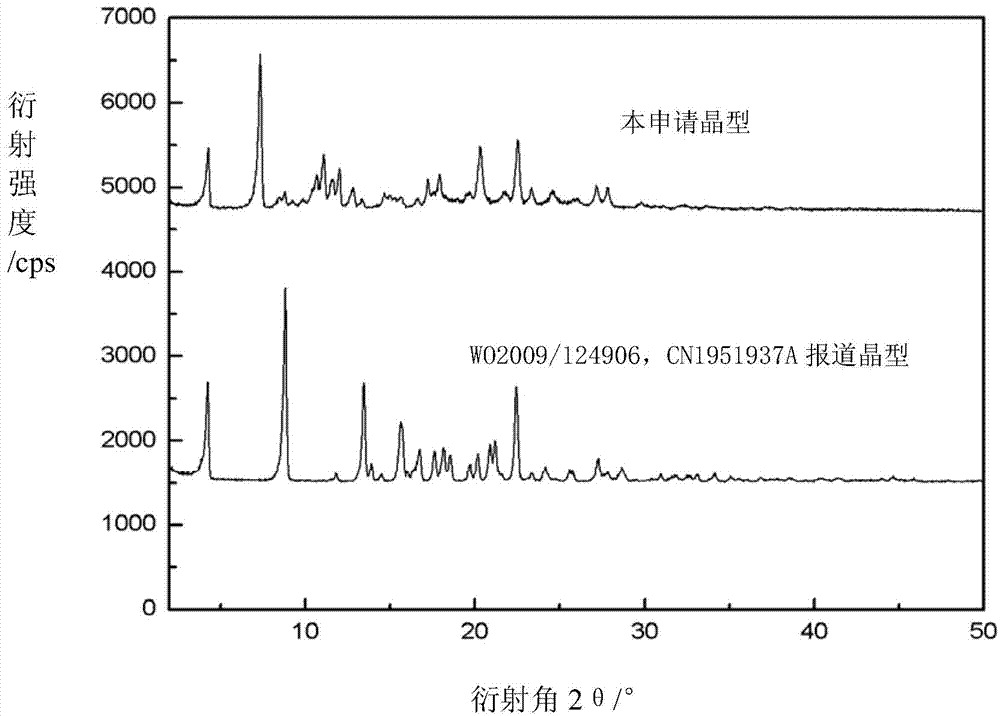
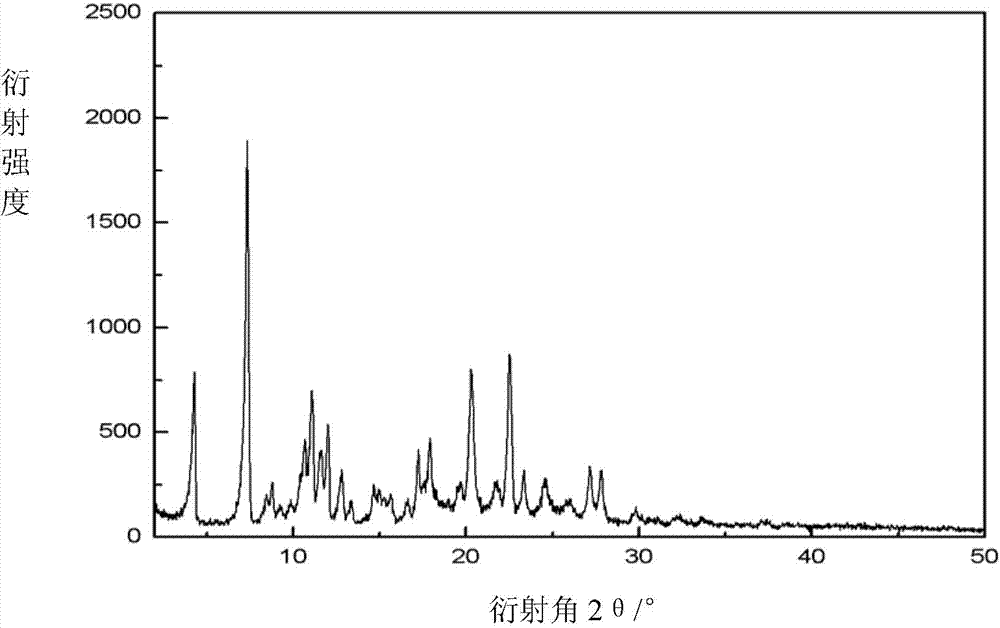
[0024] Accurately weigh 12.0g of argatroban (HPLC purity 99.0%, R / S=62.2 / 36.8) into a 250ml crystallizer, add 170ml of ethanol and 9.5ml of water to form a mixed solution, heat to 45 degrees, dissolve Keep the temperature at 45°C for one hour; slowly cool down to 5°C at 0.5°C / min, keep warm for 2-4h, after the crystallization is complete, filter and wash, and vacuum-dry at 80°C for 8h to obtain 10.3g product with a yield of 86% (HPLC The detection purity was 99.9%, R / S=62.5 / 37.4). The powder X-ray diffraction pattern of the product and figure 1 Consistent, the water content is 3.8%, and the ratio of R / S meets the medical requirements.
Embodiment 2
[0026] Accurately weigh 14.0g argatroban (HPLC purity 99.0%, R / S=62.2 / 36.8) into a 250ml crystallizer, add a mixed solution composed of 130ml ethanol and 10ml water, heat and stir until dissolved, the temperature is about at 50°C, keep the constant temperature for 10min, slowly cool down to 10°C at 0.2°C / min, and keep warm for 2-4h. After the crystallization is complete, filter and wash, and vacuum-dry at 90°C for 7h to obtain 11.3g of product with a yield of 81% (HPLC The detection purity was 99.9%, R / S=62.6 / 37.3). The powder X-ray diffraction pattern of the product and figure 1 Consistent, the water content is 3.5%, and the ratio of R / S meets the medical requirements.
Embodiment 3
[0028] Accurately weigh 30.0g of argatroban (HPLC purity 99.0%, R / S=61.8 / 37.2) into a 400ml crystallizer, add 260ml of ethanol and 10ml of water to form a mixed solution, heat and reflux to 75 degrees, and wait until the solid After complete dissolution, cool slowly at 0.2°C / min to 15°C, keep warm for 2-4h, filter and wash after complete crystallization, and vacuum-dry at 100°C for 6h to obtain 26.1g of solid with a yield of 87% (purity detected by HPLC 99.9%, R / S=61.4 / 38.5), the powder X-ray diffraction pattern of the product and figure 1 Consistent, the water content is 3.4%, and the ratio of R / S meets the medical requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com