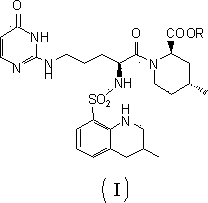
Argatroban analogue and preparation method and medical application thereof
A technology of argatroban and analogs, applied in the field of medicinal chemistry, can solve the problems of local pain, patient's physical and mental pain, and intravenous drip taking a long time, and achieve the effect of good dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: (2R,4R)-4-methyl-1-[N2-(R,S)-3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl-L-arginine Preparation of 1-Acetoxyethyl Cytosine]-2-Piperidinecarboxylate
[0043]
[0044]1. Preparation of sodium salt of ethyl hydroxyacrylate
[0045] Ethyl acetate (600ml, 6mol) was added to a 2000ml reaction flask, cooled to 0°C-5°C, sodium methoxide (220g, 4mol) was added, ethyl formate (380ml, 4.8mol) was slowly added dropwise, the dropwise addition was completed, and the temperature was raised Stir and reflux for 3 h, evaporate the solvent under reduced pressure to obtain 413 g of yellow powder, which is directly used in the next step.
[0046] 2. (2R, 4R)-4-methyl-1-[N2-(R, S)-3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl-L-arginine Preparation of Aminoisocytosine]-2-Piperidinecarboxylic Acid
[0047] Suspend 27.6g (0.2mol) of the above yellow powder in 500ml of toluene, stir, add 110g (0.195mol) of argatroban, stir and reflux for 8h, cool to room temperature, add...
Embodiment 2
[0054] Example 2: (2R,4R)-4-methyl-1-[N2-(R,S)-3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl-L-arginine Preparation of Cytosinyl]-2-piperidinecarboxylic acid-1-(isopropoxycarbonyloxy)-ethyl ester
[0055]
[0056] The title compound was synthesized in the same manner as in 3 in Example 1, using 1-iodoisopropoxycarbonylethane instead of 1-acetoxy-1-bromoethane. Yield 72.6%, HPLC content 98.2%.
[0057]
[0058] 1 H—NMR (500MHz, CDCl 3 / TMS, ppm): a total of 46 hydrogen signals
[0059] δ: 8.0 (s, 1H, NH); 5.29 (s, 1H, NH); 7.73; (s, 1H, NH); 2.1 (s, 1H, NH); 4.17 (t, 1H, JJ=7.5); 3.29~3.39 (m, 2H); 2.91~3.16 (m, 2H); 6.98~7.46 (m, 3H); 2.28 (m, 1H); 1,68 (m, 1H); 1.69~1.94 (m, 2H) ); 1.34~1.59 (m, 2H); 2.39~2.63 (m, 2H); 5.09 (m, H); 3.57 (m, H); 2.65 (t, 2H, JJ=7.1); 0.96 (d, 6H , JJ=6.8); 1.80 (t, 2H, JJ=7.1); 1.55~1.59 (m, 2H); 1.32 (d, 6H, JJ=6.8); 4.36 (t, 2H, JJ=7.0); 4.39 ( t, 2H, JJ=7.0); 6.48 (d, 2H, JJ=10.9); 7.95 (d, 2H, JJ=10.9)
[0060] MS: m / z (M...
Embodiment 3
[0061] Example 3: (2R,4R)-4-methyl-1-[N2-(R,S)-3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl-L-arginine Preparation of Ethyl Cytosine]-2-Piperidinecarboxylate
[0062]
[0063] The title compound was synthesized in the same manner as in 3 in Example 1, using 2-bromopropane instead of 1-acetoxy-1-bromoethane. Yield 84.1%, HPLC content 99.1%.
[0064]
[0065] 1 H—NMR (500MHz, CDCl 3 / TMS, ppm): a total of 42 hydrogen signals
[0066] 1 H—NMR (500MHz, CDCl 3 / TMS, ppm): a total of 42 hydrogen signals
[0067] δ: 8.0 (s, 1H, NH); 5.29 (s, 1H, NH); 7.73; (s, 1H, NH); 2.1 (s, 1H, NH); 4.17 (t, 1H, JJ=7.5); 3.29~3.39 (m, 2H); 2.91~3.16 (m, 2H); 6.98~7.46 (m, 3H); 2.28 (m, 1H); 1,68 (m, 1H); 1.69~1.94 (m, 2H) ); 1.34~1.59(m, 2H); 2.39~2.63(m, 2H); 4.55~4.85(d, 2H, JJ=7.0); 3.57(m, H); 2.65(t, 2H, JJ=7.1) ;0.96 (d, 6H, JJ=6.8); 1.80 (t, 2H, JJ=7.1); 1.55~1.59 (m, 2H); 7.25 (t, 1H, JJ=6.8) 6.48 (d, 2H, JJ= 10.9); 7.95 (d, 2H, JJ=10.9)
[0068] MS: m / z (M + ) 586 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com