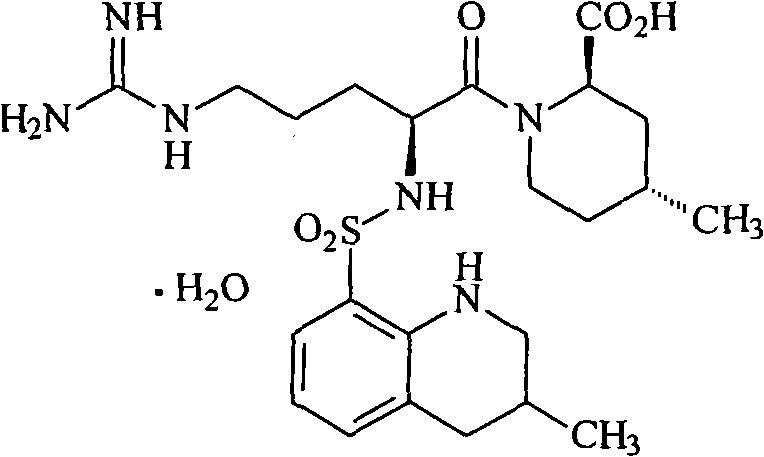
Argatroban and preparation thereof
A technology of argatroban and a synthesis method, which is applied in the field of drug synthesis, can solve the problems of complicated operation, low yield, large respiratory tract irritation, etc., and achieves the effects of simplified operation steps, simple synthesis process and shortened reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
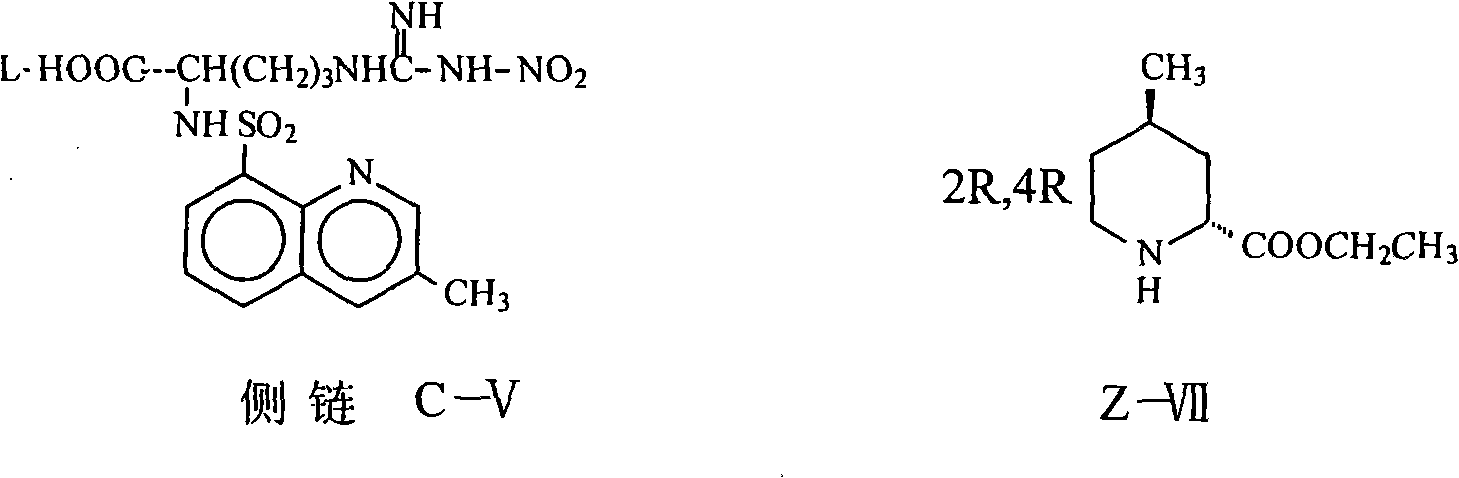
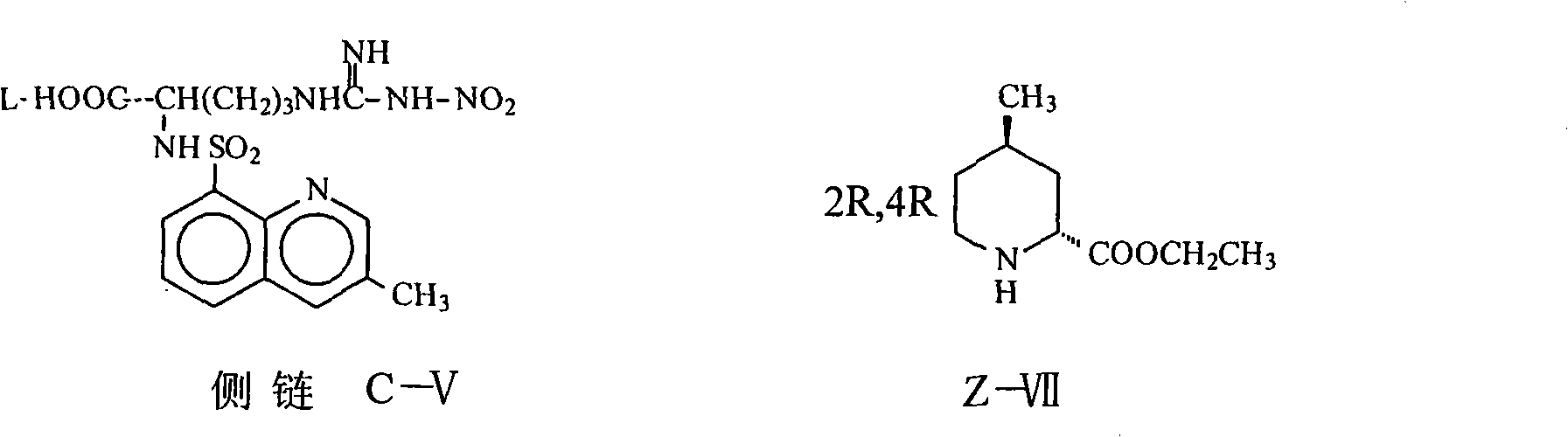
[0034] (2R, 4R)-1-[N G -Nitro-N 2 Preparation of -(3-methyl-8-quinolinesulfonyl)-L-arginyl]-4-methyl-2-piperidinecarboxylic acid ethyl ester (Z-VIII):
[0035]Add 76.4 g (0.18 mol) of carboxylate and 30.7 g (0.18 mol) of (2R, 4R) 4MPE into the reaction flask, add 1000 ml of THF and stir to dissolve, add 49.5 g (0.18 mol) of DPPA at 0°C, and stir at room temperature for 14 hours. Add 500ml of saturated brine, stir for 10 minutes, separate the organic layer, extract the aqueous layer with THF 200ml×2, combine the organic layers, concentrate to dryness, add 500ml of chloroform to dissolve, wash with sodium bicarbonate aqueous solution and water, and dry, Concentrate to dryness to obtain a yellow solid with a yield of 54%, HPLC≥92% (normalization method).
Embodiment 2
[0037] (2R, 4R)-1-[N G -Nitro-N 2 Preparation of -(3-methyl-8-quinolinesulfonyl)-L-arginyl]-4-methyl-2-piperidinecarboxylic acid ethyl ester (Z-VIII):
[0038] Add 100g (0.24 moles) of the carboxylate and 44g (0.26 moles) of (2R, 4R) 4MPE into the reaction flask, add 1000ml of dichloromethane and stir to dissolve, add 72.6g (0.26 moles) of diethyl bromophosphate at -5°C 23.2ml (0.288mol) of pyridine was added dropwise under stirring, stirred at 30°C for 6 hours, 500ml of saturated saline was added, the organic layer was separated, the aqueous layer was extracted with 400ml of dichloromethane, the organic layers were combined, and sodium bicarbonate aqueous solution and Washed with water, dried and concentrated to dryness to obtain a yellow solid with a yield of 84.5%, HPLC≥95% (normalization method).
Embodiment 3
[0040] (2R, 4R)-1-[N G -Nitro-N 2 Preparation of -(3-methyl-8-quinolinesulfonyl)-L-arginyl]-4-methyl-2-piperidinecarboxylic acid ethyl ester (Z-VIII):
[0041] Add 76.4g (0.18 moles) of the carboxylate and 30.7 grams (0.18 moles) of (2R, 4R) 4MPE to the reaction flask, add 800ml of DMF, stir to dissolve, add 31.2ml (0.216 moles) of diethyl phosphate chloride at 0°C, Add 26.4 g (0.216 mol) of DMAP under stirring, stir at 25°C for 6 hours, evaporate the solvent to dryness under reduced pressure, add 500 ml of water, extract the aqueous layer three times with 600 ml of dichloromethane, combine the organic layers, and use sodium bicarbonate aqueous solution and water respectively Washed, dried, and concentrated to dryness to obtain a yellow solid with a yield of 88.5%, HPLC≥95% (normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com