Preparation method of optically-pure argatroban
An argatroban and optical technology, applied in the field of medicine, can solve the problems of unfavorable production safety and labor protection, high price of quinoline sulfonyl chloride, complicated separation process, etc., and achieves low production cost, high ee value and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
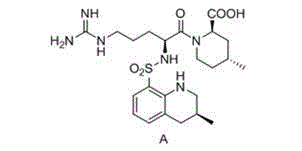
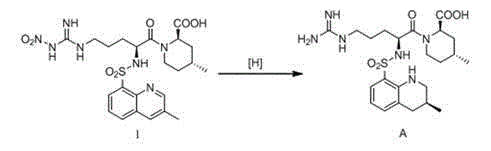
[0037] Embodiment 1 Preparation of formula A argatroban
[0038] Add 27.5g (0.05mol) of the compound of formula I and 150mL of methanol into a 250mL three-necked flask, stir to dissolve, add 38.6mg (0.05mmol) of the chiral catalyst shown in formula II and 25mL of formic acid, under nitrogen protection, react at 20°C for 3~ 5h, TLC monitors the reaction. After the reaction, the catalyst was removed by filtration, the filter cake was washed with an appropriate amount of methanol, the filtrates were combined, and concentrated to dryness under reduced pressure to obtain about 23.7 g of a yellow solid.
[0039] Add the obtained solid into a 250mL three-necked flask, add 30mL of ethanol and 100mL of purified water, heat to reflux to dissolve, then add 0.2g of activated carbon, continue to reflux for 30min, filter to remove the activated carbon while it is hot, and naturally cool and crystallize the filtrate, filter, wash, 80 After vacuum drying at ℃ for 5 hours, 22.6 g of off-whit...
Embodiment 2
[0040] Embodiment 2 Preparation of formula A argatroban
[0041]Add 27.5g (0.05mol) of the compound of formula I and 150mL of methanol into a 250mL three-necked flask, stir to dissolve, add 19.3mg (0.025mmol) of the chiral catalyst shown in formula II and 25mL of formic acid, under nitrogen protection, react at 30°C for 3~ 5h, TLC monitors the reaction. After the reaction, the catalyst was removed by filtration, the filter cake was washed with an appropriate amount of methanol, the filtrates were combined, and concentrated to dryness under reduced pressure to obtain about 23.0 g of a yellow solid.
[0042] Add the obtained solid into a 250mL three-necked flask, add 30mL of ethanol and 100mL of purified water, heat to reflux to dissolve, then add 0.2g of activated carbon, continue to reflux for 30min, filter to remove the activated carbon while it is hot, and naturally cool and crystallize the filtrate, filter, wash, 80 After vacuum drying at ℃ for 5 hours, 21.6 g of white s...
Embodiment 3
[0043] Embodiment 3 Preparation of formula A argatroban
[0044] Add 27.5g (0.05mol) of the compound of formula I and 150mL of methanol into a 250mL three-necked flask, stir to dissolve, add 45.2mg (0.05mmol) of the chiral catalyst shown in formula III and 2.5g of ammonium formate, nitrogen protection, and react at 30°C 3~5h, TLC monitors the reaction. After the reaction, the catalyst was removed by filtration, the filter cake was washed with an appropriate amount of methanol, the filtrates were combined, and concentrated to dryness under reduced pressure to obtain about 22.8 g of a yellow solid.
[0045] Add the obtained solid into a 250mL three-necked flask, add 35mL of ethanol and 100mL of purified water, heat to reflux to dissolve, then add 0.2g of activated carbon, continue to reflux for 30min, filter while hot to remove the activated carbon, the filtrate is naturally cooled and crystallized, filtered, washed, 80 After vacuum drying at ℃ for 5 hours, 20.9 g of off-whit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com