Controlled drug release composition and drug releasing medical device
A release-controlled, medical device technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3、 comparative example 1~3
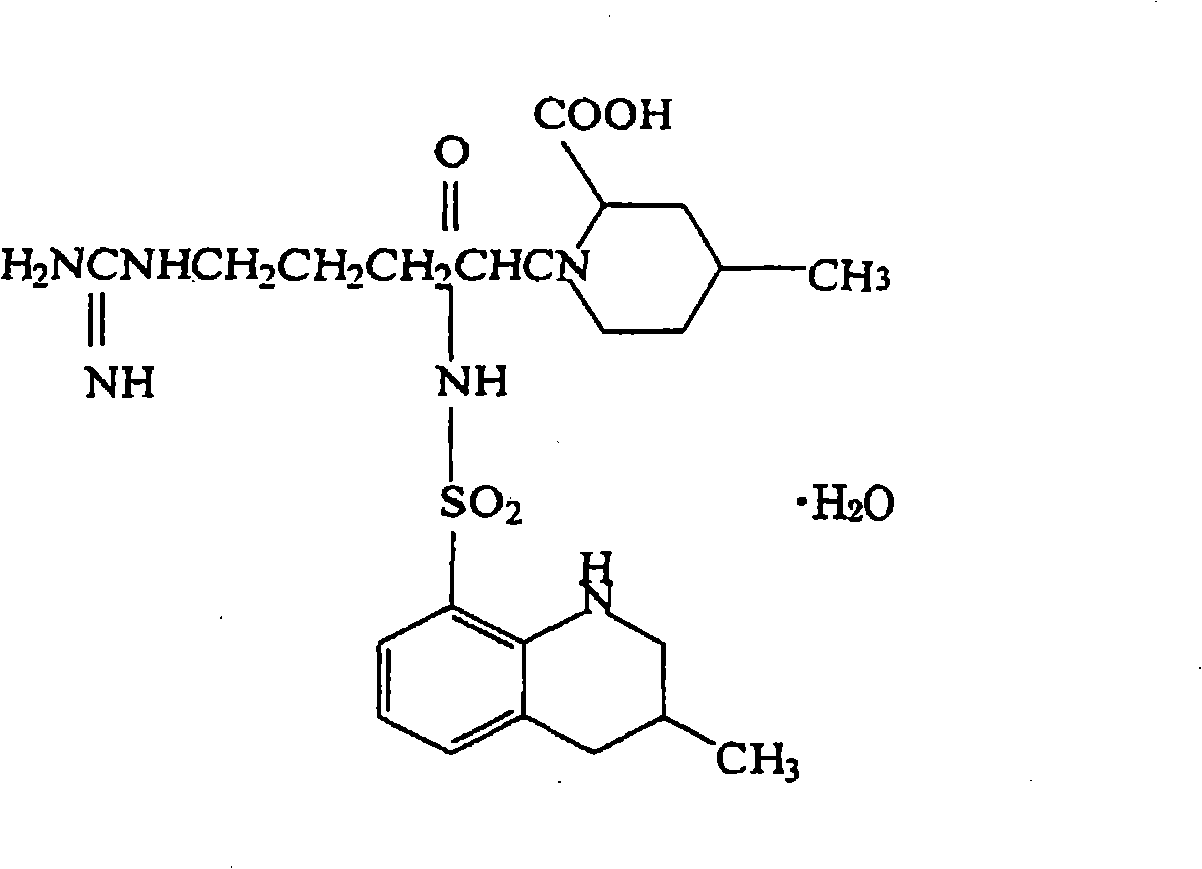
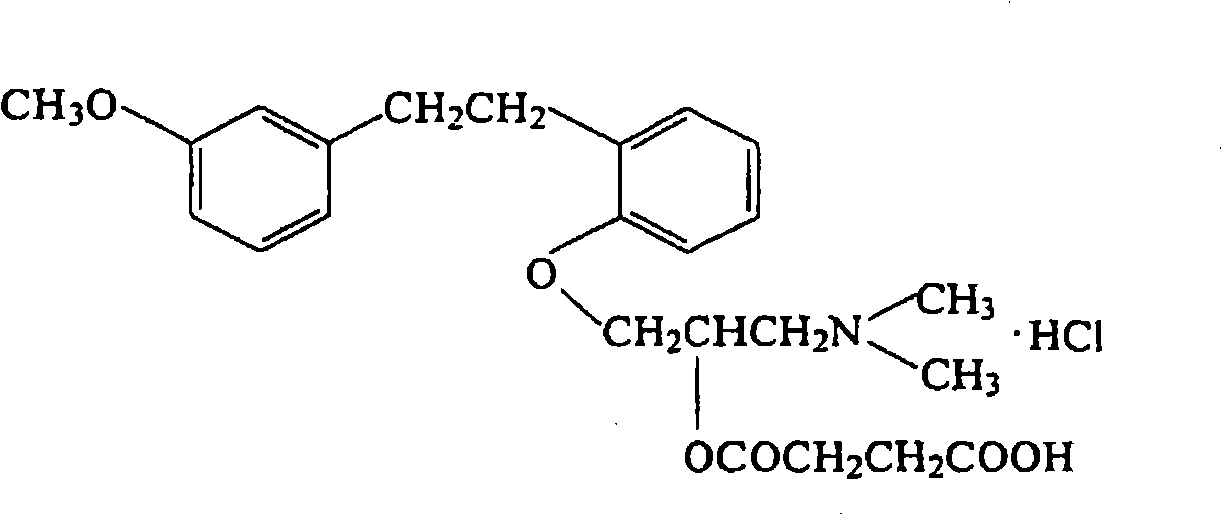
[0138] As shown in Table 1, dissolve 90 mg of polylactic acid and lactic acid / glycolic acid copolymer, 10 mg of triethyl citrate as a release aid, and 10 mg of sarcogrelate hydrochloride as an antiplatelet drug in 1 mL of hexafluoroisopropanol , cast on a glass dish with a diameter of 41 mm, and air-dry to obtain a drug carrier. Soak it in 100 mL of pH 7.4 phosphate buffer, sample the buffer regularly, and measure the absorbance (Abs) at 270 nm as the characteristic absorption band of sarcogrelate hydrochloride, thereby tracking the amount of dissolution of the drug. Table 1 shows the absorbance values from the start of dissolution to 3 weeks after.
[0139] As Comparative Examples 1 to 3, the same dissolution test was performed under the same conditions as in the Examples except that no release aid was added.
[0140] [Table 1]
[0141] polymer
Embodiment 4~6、 comparative example 4
[0143] Dissolve 90 mg of lactic acid / glycolic acid (50 / 50) copolymer, 10 mg of alkyl tartrate diester as a release aid, and 10 mg of sarcogrelate hydrochloride as an antiplatelet drug in 1 mL of hexafluoroisopropanol. Cast on a glass dish with a diameter of 41mm, and air-dry to obtain the drug carrier. Soak it in 100 mL of pH 7.4 phosphate buffer, sample the buffer regularly, and measure the absorbance at 270 nm, which is the characteristic absorption band of sarcogrelate hydrochloride, so as to track the amount of dissolution of the drug. Table 2 shows the absorbance values from the start of the dissolution to 3 weeks later.
[0144] [Table 2]
[0145] release aid
Embodiment 7~10、 comparative example 5、6
[0147] As shown in Table 3, 90 mg of polylactic acid and lactic acid / glycolic acid copolymer, 10 mg of diethyl tartrate or triethyl citrate as a release aid, and 10 mg of argatroban as an antithrombin drug were dissolved in Cast in 1 mL of hexafluoroisopropanol on a glass dish with a diameter of 41 mm, and air-dry to obtain the drug carrier. It was soaked in 100 mL of pH 7.4 phosphate buffer solution, the buffer solution was regularly sampled, and the absorbance value at 330 nm, which is the characteristic absorption band of argatroban, was measured to track the dissolution amount of the drug. Table 3 shows the absorbance values from the start of the dissolution to 3 weeks afterward.
[0148] As Comparative Examples 5 and 6, the same dissolution test was performed under the same conditions as in the Examples except that no release aid was added.
[0149] [table 3]
[0150] Polymer (90mg)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com