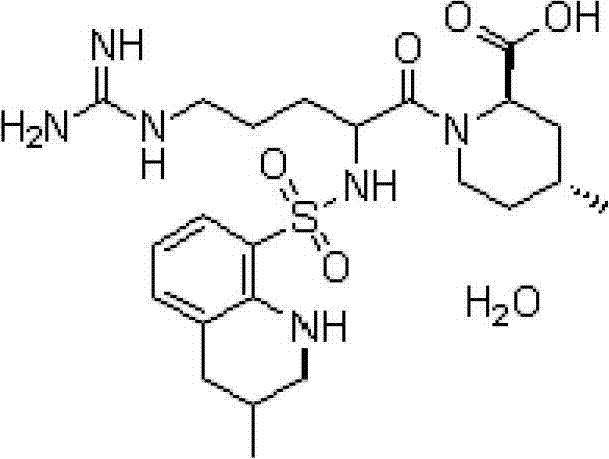
Argatroban drug composition and preparation method and application of argatroban drug composition
A technology of argatroban and its composition, which is applied in the field of argatroban pharmaceutical composition and its preparation, can solve the problems of large pH fluctuations before and after sterilization of injections, weak buffering capacity of argatroban, and increased clinical use Risk and other issues, to achieve the effect of increasing clinical safety, short preparation time, and suitable for clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] prescription:
[0067]
[0068] Weigh 30 g of absolute ethanol and 50 g of propylene glycol, stir evenly to form a stable solvent system, add 1 g of the main drug argatroban in the prescribed amount, stir to fully dissolve the main drug, and the solution becomes clear, add water for injection to dilute to 200 ml, Mix evenly to obtain a preparation solution. After the intermediate is qualified, the solution is filtered, filled into packaging containers, and terminally sterilized by moist heat to obtain the final pharmaceutical composition.
[0069] The content of the finished product is 100.3% by HPLC, the total impurities are 0.056%, the maximum simple impurities are 0.014%, the pH value before sterilization is 6.68, and the pH value after sterilization is 6.57. The content and related substances are detected by HPLC.
Embodiment 2
[0071] prescription:
[0072]
[0073] Weigh 20 g of absolute ethanol and 40 g of propylene glycol, stir evenly to form a stable solvent system, add 1 g of the main drug argatroban in the prescribed amount, stir to fully dissolve the main drug, and the solution becomes clear, add water for injection to dilute to 200 ml, Mix evenly to obtain a preparation solution. After the intermediate is qualified, the solution is filtered, filled into packaging containers, and terminally sterilized by moist heat to obtain the final pharmaceutical composition.
[0074] The content of the finished product is 101.3% by HPLC, the total impurity is 0.068%, the maximum simple impurity is 0.014%, the pH value before sterilization is 6.73, and the pH value after sterilization is 6.61.
Embodiment 3
[0076] prescription:
[0077]
[0078] Weigh 50 g of absolute ethanol and 40 g of propylene glycol, stir evenly to form a stable solvent system, add 1 g of the main drug argatroban in the prescribed amount, stir to fully dissolve the main drug, and the solution becomes clear, add water for injection to dilute to 200 ml, Mix evenly to obtain a preparation solution. After the intermediate is qualified, the solution is filtered, filled into packaging containers, and terminally sterilized by moist heat to obtain the final pharmaceutical composition.
[0079] The content of the finished product is 100.8% by HPLC, the total impurity is 0.047%, the maximum simple impurity is 0.021%, the pH value before sterilization is 6.93, and the pH value after sterilization is 6.75.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com