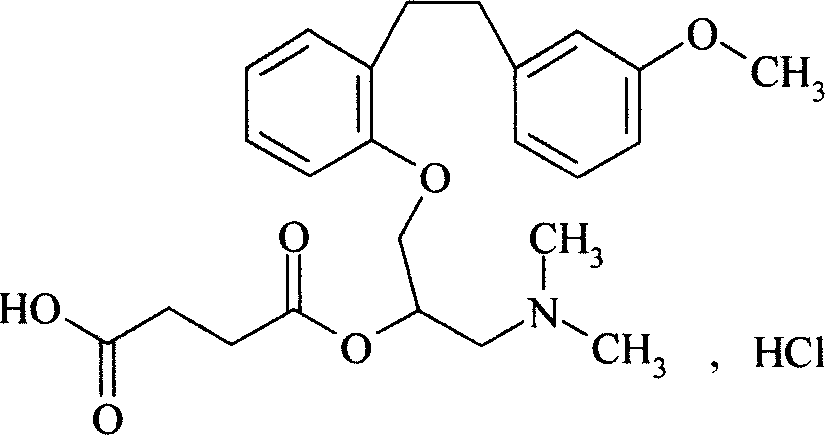
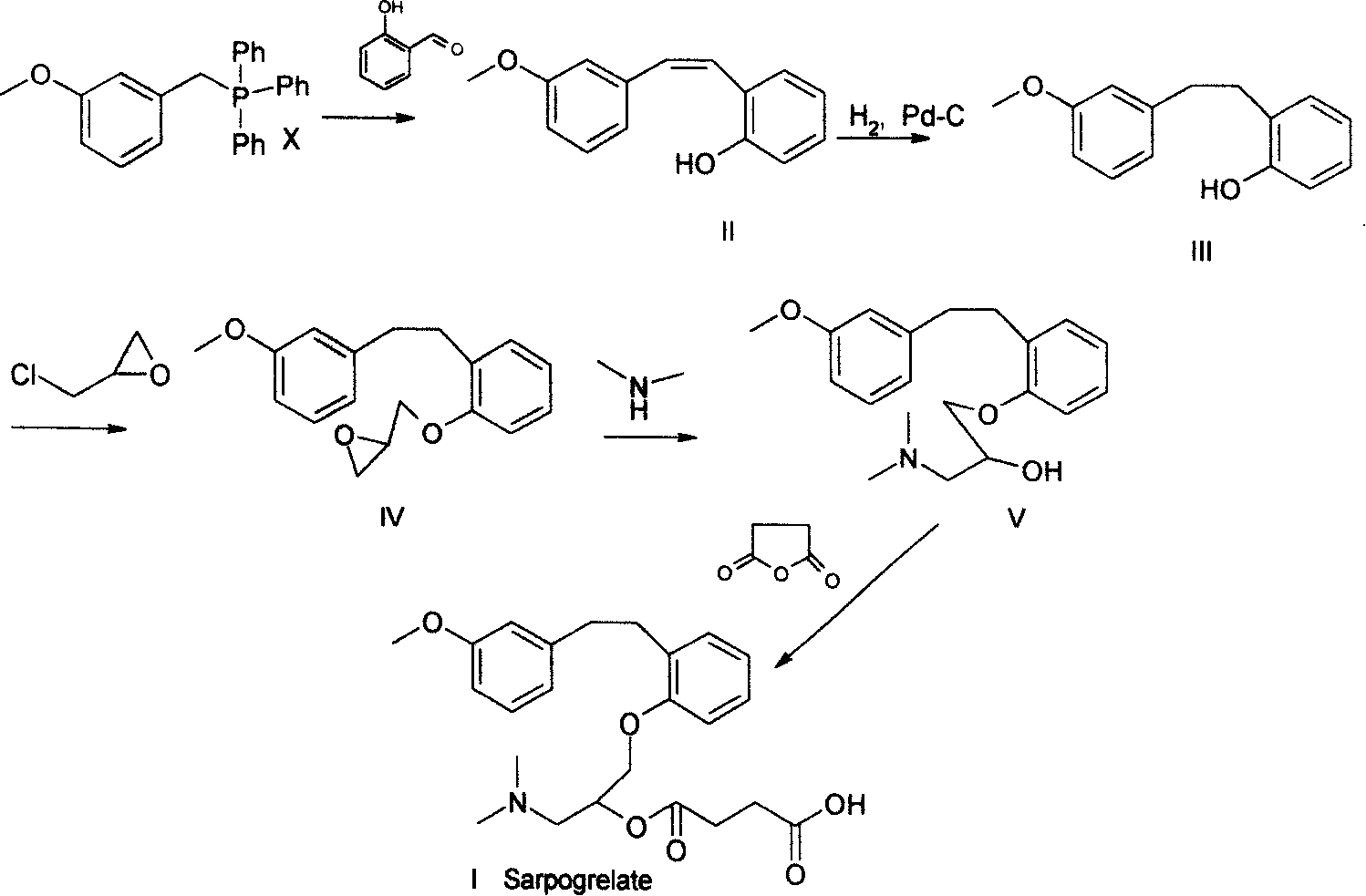
Method for preparing sarpogrelate hydrochloride
A technology for sarpogrelate hydrochloride and salt formation, which is applied in the field of preparation of sarpogrelate hydrochloride, can solve the problems of being difficult to remove, difficult to obtain high-purity products, and the like, and achieves the effects of easy operation, easy industrial production and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 2-((3-methoxy)phenethyl)phenol (mixture)
[0039] 100 kg (containing 55% of triphenylphosphine oxide) of 2-((3-methoxy) styryl) phenol mixture prepared by Wittg reaction (J.Med.Chem.1990, 33:1818-1823) Into a 1000L autoclave, add 450 liters of ethanol, 15 kg of 5% Pd-C, add hydrogen to 0.5Mpa, stir and hydrogenate at a temperature of 50°C for 3 hours, filter and concentrate to obtain a light yellow oil with a yield of 96%, HPLC Detection showed complete hydrogenation.
Embodiment 2
[0040] Example 2 2-((3-methoxy)phenethyl)phenol (mixture)
[0041] Add 190 grams of 2-((3-methoxy) styryl) phenol mixture (containing 52% triphenylphosphine oxide) prepared by Wittg reaction into the reaction flask, add 1000 ml of methanol, 20 grams of Raney nickel, Introduce hydrogen, stir and hydrogenate at 40°C for 10 hours, filter, and concentrate to obtain a light yellow oil, which is detected by HPLC and shows that the hydrogenation is complete.
Embodiment 3
[0042] Example 3 2-((3-methoxy)phenethyl)phenol (mixture)
[0043] As in Example 1, 10% palladium carbon was used instead, the pressure was 1 MPa, and the reaction was carried out at room temperature, with a yield of 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com