Preparation method of sarpogrelate hydrochloride photodegradation impurities
A technology of sarcogrelate hydrochloride and photodegradation, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxyl compounds, etc., can solve the problems of lack of literature and patent reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
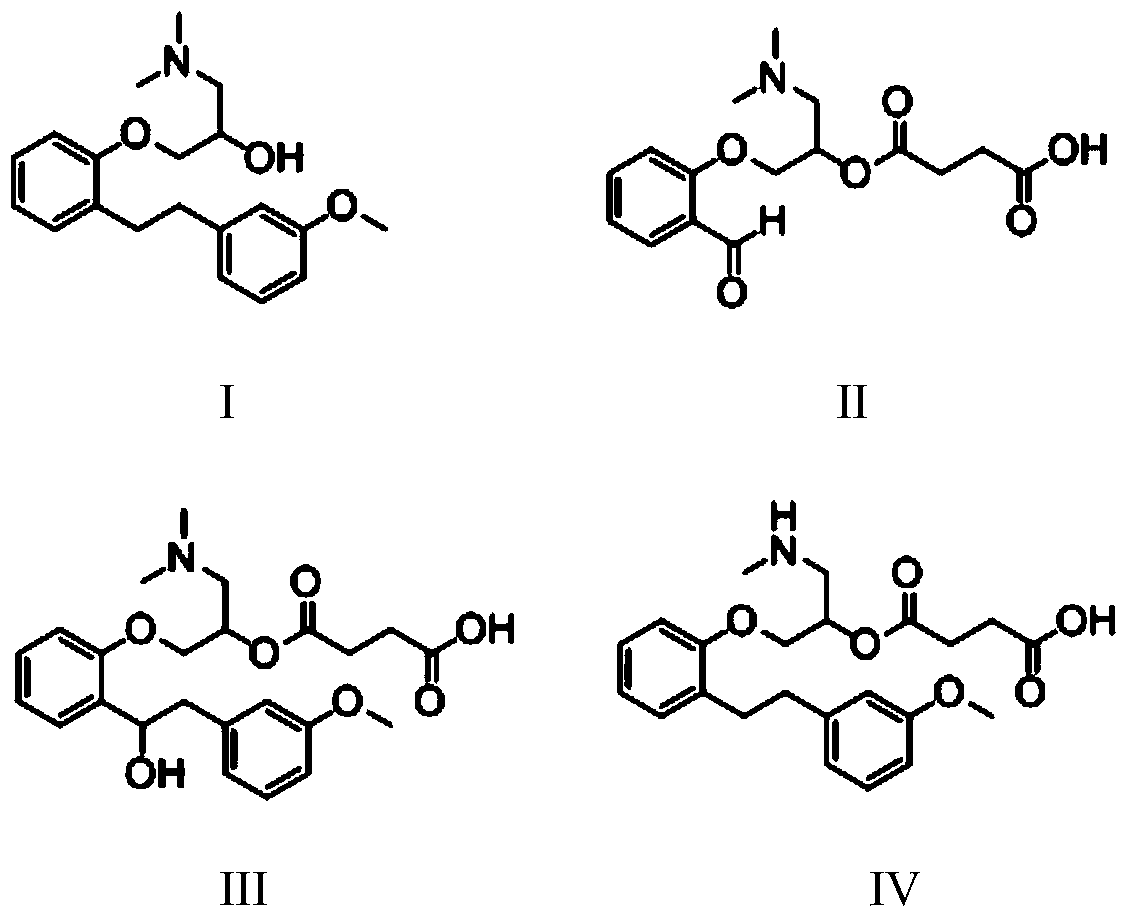
[0091] Salicylaldehyde (10.0g, 82mmol) was added to a 250ml reaction flask, 100ml of tetrahydrofuran was added, epichlorohydrin (9.1g, 98mmol) and 10g of potassium carbonate were added, under nitrogen protection, the temperature was raised to reflux, and reacted for 12h. TLC detects that the raw material has been reacted (petroleum ether: ethyl acetate=4:1v / v), and the product 2-(2-oxomethyloxirane) benzaldehyde II-2 is obtained after post-treatment, post-treatment: suction filtration Potassium carbonate was removed, and the filtrate was concentrated by distillation under reduced pressure to obtain compound 2-(2-oxomethyloxirane)benzaldehyde II-2, 10.39 g, yield 71.2%.
Embodiment 2
[0093] Salicylaldehyde (10.0g, 82mmol) was added to a 250ml reaction flask, 100ml of tetrahydrofuran was added, epichlorohydrin (9.1g, 98mmol) and 5g of potassium hydroxide were added, under nitrogen protection, the temperature was raised to reflux, and reacted for 15h. TLC detects that the raw material has been reacted (petroleum ether: ethyl acetate=4:1v / v), and the product 2-(2-oxomethyloxirane) benzaldehyde II-2 is obtained after post-treatment, post-treatment: suction filtration Potassium carbonate was removed, and the filtrate was concentrated by distillation under reduced pressure to obtain compound 2-(2-oxomethyloxirane)benzaldehyde II-2, 8.76 g, yield 60.0%.
Embodiment 3
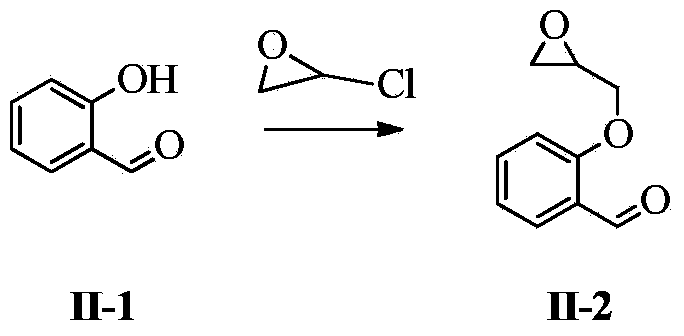
[0095] Compound 2-(2-oxomethyloxirane)benzaldehyde (10.0g, 60mmol) was dissolved in 100ml of tetrahydrofuran, cooled to 0°C, added 10g of 40% dimethylamine aqueous solution, and reacted at room temperature for 4h, TLC Tracking (petroleum ether: ethyl acetate = 4:1 v / v). Concentrate after completion of the reaction, extract with ethyl acetate (100*3ml), dry the organic phase over anhydrous sodium sulfate, and concentrate to obtain 2-[2-(dimethylamino)-1-hydroxyethoxy]benzaldehyde II- 3, 11.22 g, yield 89.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com