Sarpogrelate hydrochloride sustained release pellet and preparation method thereof
A technology of sarpogrelate hydrochloride and sustained-release pellets is used in pharmaceutical formulations, medical preparations containing active ingredients, and devices for making medicines into special physical or taking forms, etc. problems such as pellet processing properties, to achieve the effect of low friability, controllable quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the main factor that affects the forming of pill core containing medicine
[0084] In this example, in the case of the selected drug, the solubility of the active ingredient (vitamin C (0.2g / ml, easily soluble), sarcogrelate hydrochloride (0.02g / ml, slightly soluble)), the excipient (microcrystalline cellulose), wetting agent (20% ethanol), surfactant (polysorbate 80), wetting time of soft material (min), extrusion frequency (HZ), sieve aperture (mm), 9 variables such as spheronization time (min), drying temperature (°C), and 2 dummy variables (Dummy) were designed in P-B experiment (N=12) to investigate the main factors affecting the formation of drug-containing pellet cores. Wherein, the amount of the active ingredient is the weight ratio of the active ingredient to (the sum of the active ingredient and the excipient), and the amount of the excipient is the weight ratio of the excipient to the (the sum of the active ingredient and the excipient). The am...
Embodiment 2
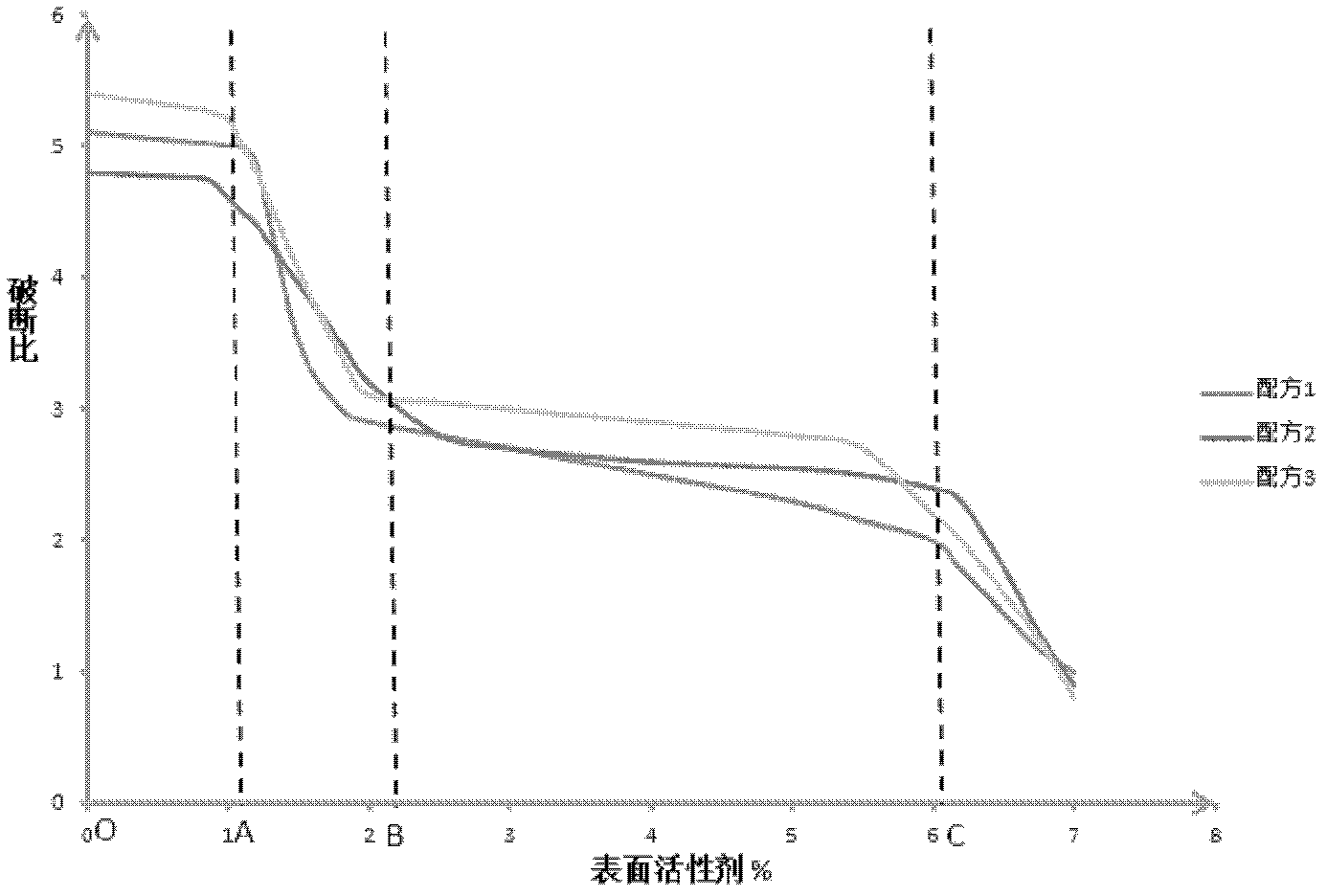
[0097] Embodiment 2: Surfactant is to the influence of important procedure in the processing of core containing pill
[0098] In this embodiment, vitamin C (easy to dissolve), taurine (dissolved), and bromamide hydrochloride (slightly soluble) were respectively used as active ingredients with microcrystalline cellulose (excipient), 20% ethanol (moisturized wet agent) to form the basic formula, adding and (active substance+excipient) weight percent is 0, 1, 2, 3, 4, 6, 7% surfactant (polysorbate 80 ) to form a series of formulas (the active ingredient of formula 1 is vitamin C, the active ingredient of formula 2 is taurine, and the active ingredient of formula 3 is bromamide hydrochloride), according to the "preparation method" and "process parameters" in the "example description" Prepare the bar, observe and calculate the shape of the bar and the yield per unit time, and drop the bar naturally into a 1-meter square plate, shake it gently and transfer it to an electric blast dr...
Embodiment 3
[0112] Containing pill core formula: parts by weight
[0113] Active ingredient: 79.5 parts of sarcogrelate hydrochloride
[0114] Excipient 1: 20 parts of microcrystalline fiber
[0115] Excipient 2: Hypromellose 0.5 parts
[0116] Surfactant: 2.5 parts of polyoxyethylene (40) hydrogenated castor oil
[0117] Wetting agent: 20% ethanol 20 parts
[0118] Coating solution formula (according to 100 parts by weight containing pill core):
[0119] 10% Surelease Water dispersion 100 parts
[0120] Note: 10% Surelease Aqueous dispersion Ethyl cellulose aqueous dispersion.
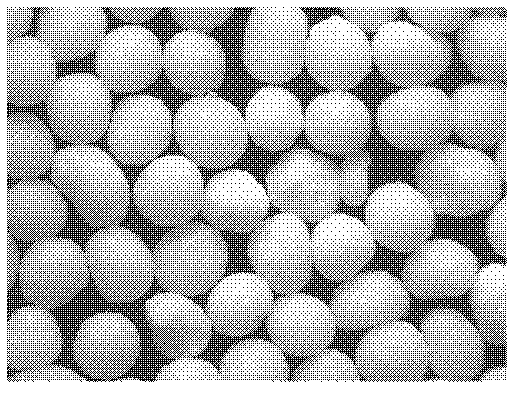
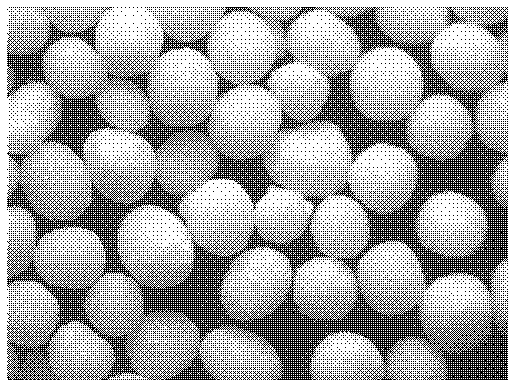
[0121] Preparation process: Prepare three batches of micropills according to the "Example Description" and the above formula. The quality evaluation results are shown in Table 6 below, and the properties of the micropills are shown in Table 6. diagram 2-1 , 2-2 , 2-3.
[0122] Table 6: Ball core quality evaluation
[0123] Evaluation index / batch
[0124] Comprehensive form factor (e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com