Sarpogrelate hydrochloride single layer osmotic pump regulated-release preparations and preparation thereof
The technology of sarpogrelate hydrochloride and single-layer osmotic pump, which is applied in the field of medicine, can solve the problems of reduced medication frequency, large fluctuation of blood drug concentration, easy accumulation of drugs, etc., and achieves the effects of less toxic and side effects, lasting effect and stable curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
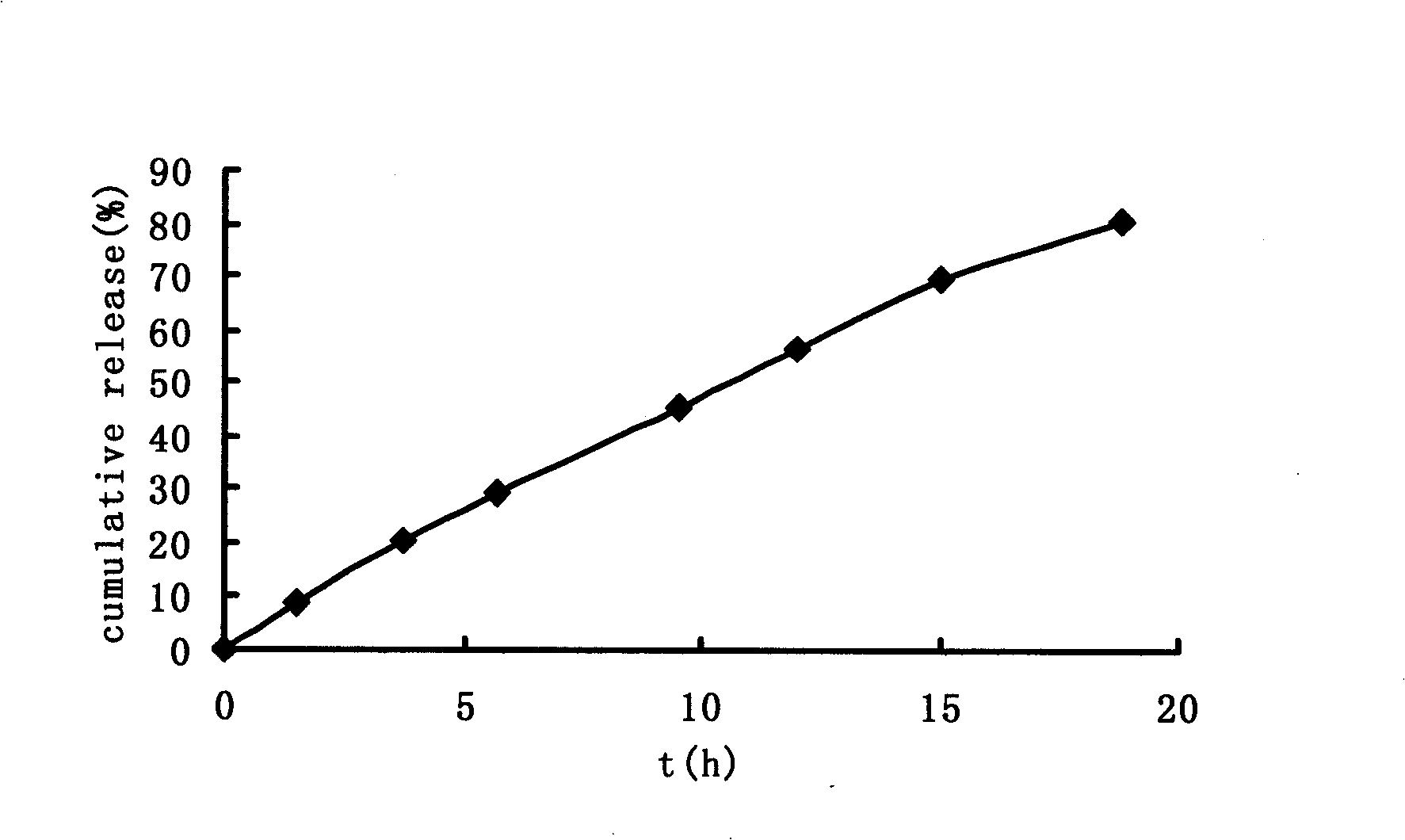
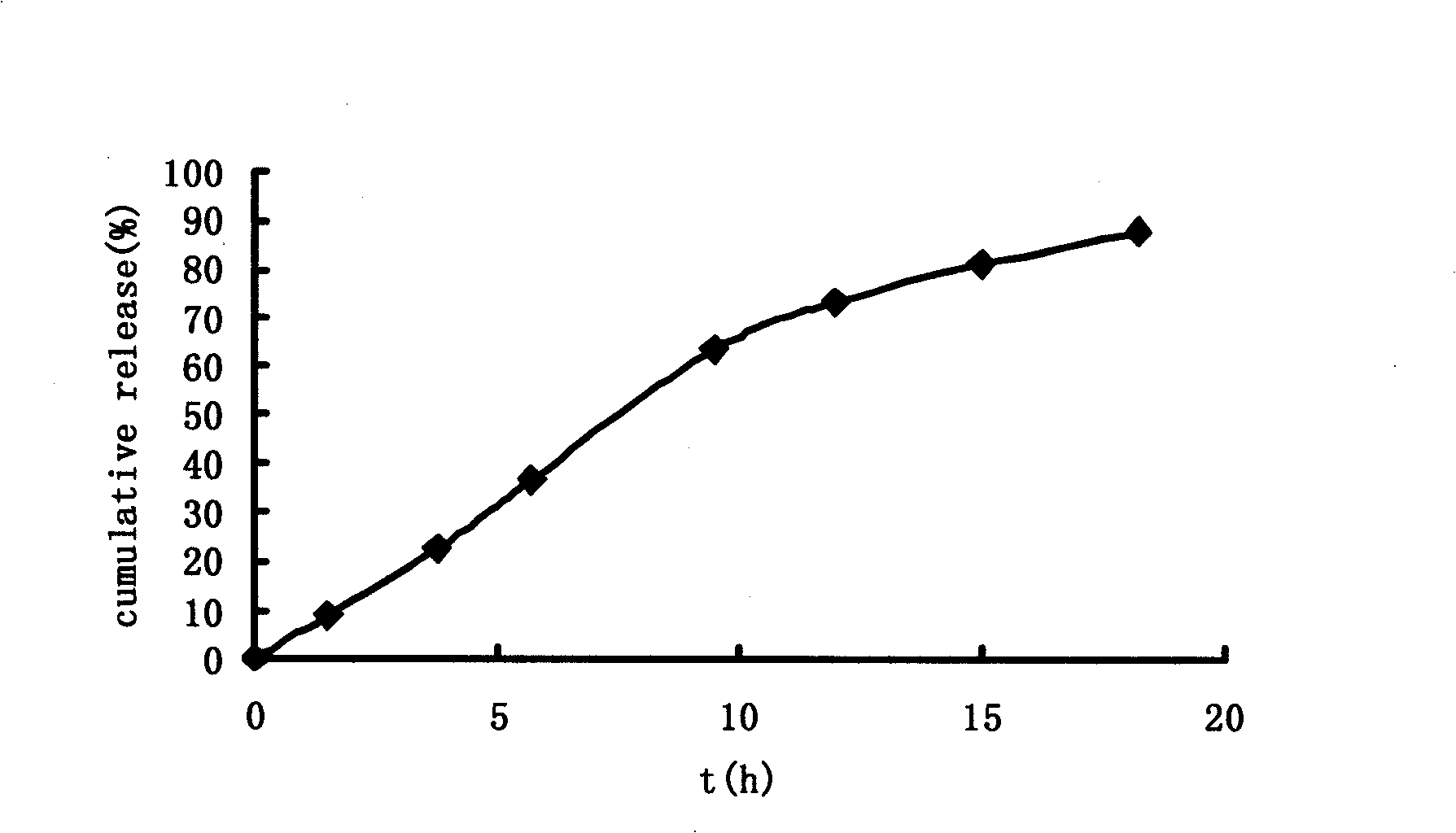
Image
Examples
Embodiment 1
[0024] Chip composition:
[0025] Sagrelate Hydrochloride 25%
[0026] Polyoxyethylene (molecular weight: 300,000) 42.5%
[0027] Citric acid 32.0%
[0028] Magnesium Stearate 0.5%
[0029] Composition of semi-permeable membrane coating solution:
[0030] Cellulose acetate 2.0%
[0031] Macrogol 400 0.16%
[0033] Preparation method: crush the prescription amount of medicine, citric acid and polyoxyethylene respectively, pass through a 100-mesh sieve, mix well, soften with starch slurry, pass through 18-mesh granulation, oven-dried at 60°C, pass through a 20-mesh sieve Granules, add lubricant, mix evenly, and directly compress into tablets to obtain tablet cores. Dissolve cellulose acetate and plasticizer PEG400 in acetone solution as a coating solution, put the tablet core in a coating pan, and coat at a coating temperature of 40-60°C. After coating, coat the tablet at 40 Dry in a drying oven at ℃ to solidify the coating film, and then prepare...
Embodiment 2
[0035] Chip composition:
[0036] Sagrelate Hydrochloride 20%
[0037] Polyoxyethylene (molecular weight: 300,000) 15%
[0038] Citric acid 64.5%
[0039] Magnesium Stearate 0.5%
[0040] Composition of semi-permeable membrane coating solution:
[0041] Cellulose acetate 2.5%
[0042] Macrogol 400 0.25%
[0044]Preparation method: crush the prescription amount of medicine, citric acid and polyoxyethylene respectively, pass through a 100-mesh sieve, mix well, soften with starch slurry, pass through 18-mesh granulation, oven-dried at 60°C, pass through a 20-mesh sieve Granules, add lubricant, mix evenly, and directly compress into tablets to obtain tablet cores. Dissolve cellulose acetate and plasticizer PEG400 in acetone solution as a coating solution, put the tablet core in a coating pan, and coat at a coating temperature of 40-60°C. After coating, coat the tablet at 40 Dry in a drying oven at ℃ to solidify the coating film, and then prepare a ...
Embodiment 3
[0046] Chip composition:
[0047] Sagrelate Hydrochloride 30%
[0048] Polyoxyethylene (molecular weight: 300,000) 50%
[0049] Lactose 19.5%
[0050] Magnesium Stearate 0.5%
[0051] Composition of semi-permeable membrane coating solution:
[0052] Cellulose acetate 3.0%
[0053] Macrogol 400 0.3%
[0054] Acetone: isopropanol (4:1) solvent
[0055] Preparation method: respectively pulverize the prescribed amount of medicine, lactose and polyoxyethylene through a 100-mesh sieve, mix uniformly, then add lubricant, mix uniformly, and directly compress into tablets to obtain tablet cores. Dissolve cellulose acetate and plasticizer PEG 400 in acetone solution as a coating solution, put the tablet core in a coating pan, and coat at a coating temperature of 40-60°C. After coating, coat the tablet in the Dry in a drying oven at 40°C to solidify the coating film, and then prepare a 0.5mm drug release hole on one side of the coating tablet by laser or mechanical means to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com