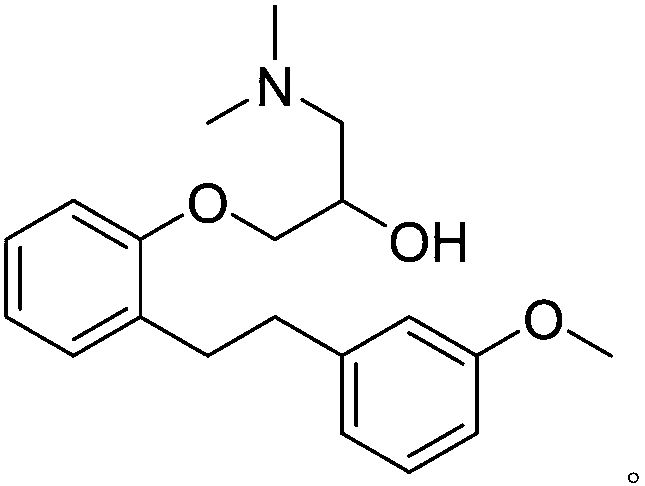
Use of sarpogrelate hydrochloride impurities I for inducing differentiation of bone marrow mesenchymal stem cells
A technology of sarcogrelate hydrochloride and bone marrow mesenchyme, which is applied in the field of inducing bone marrow mesenchymal stem cell differentiation, sarcogrelate hydrochloride impurities, and can solve the problem of no pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
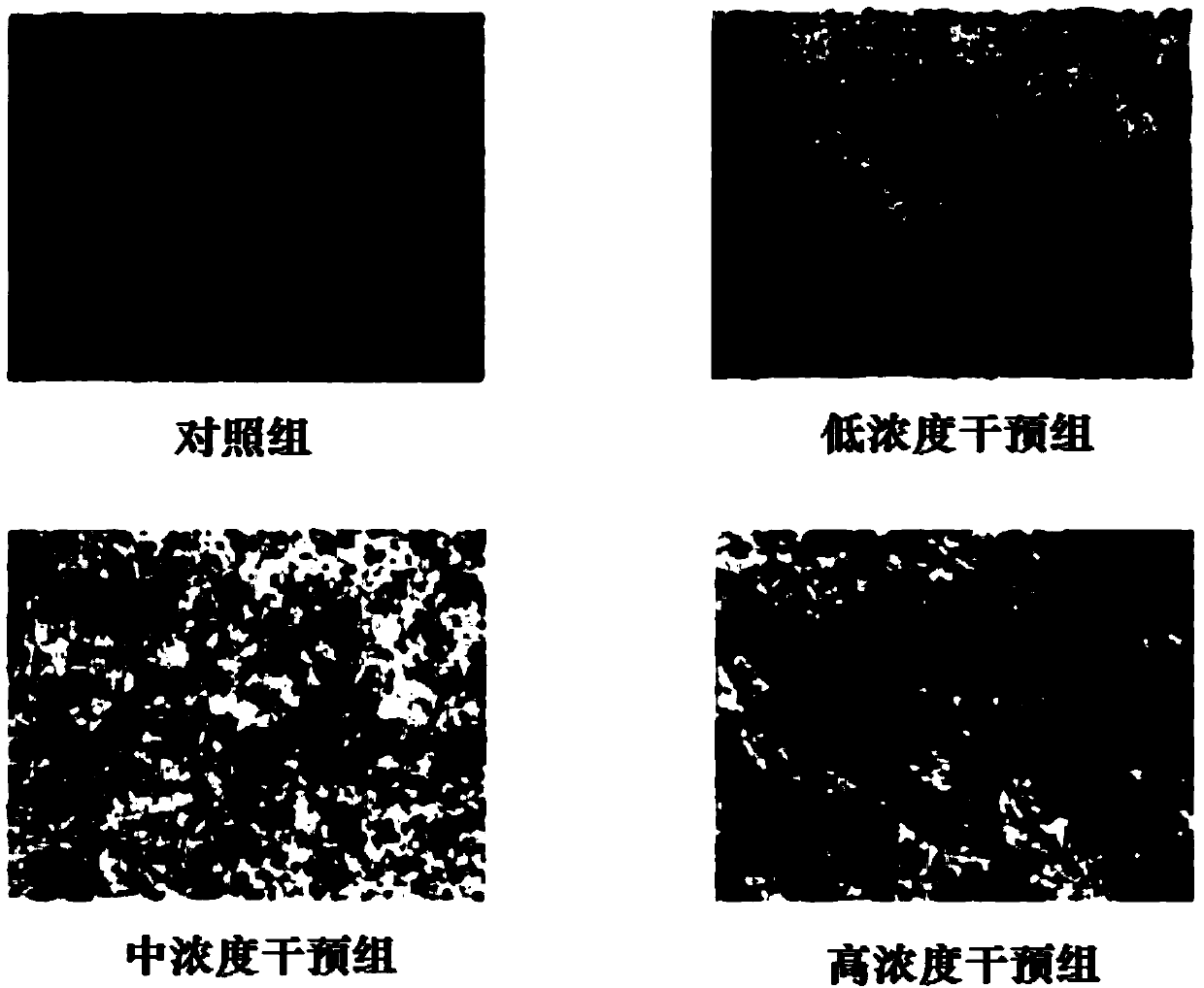
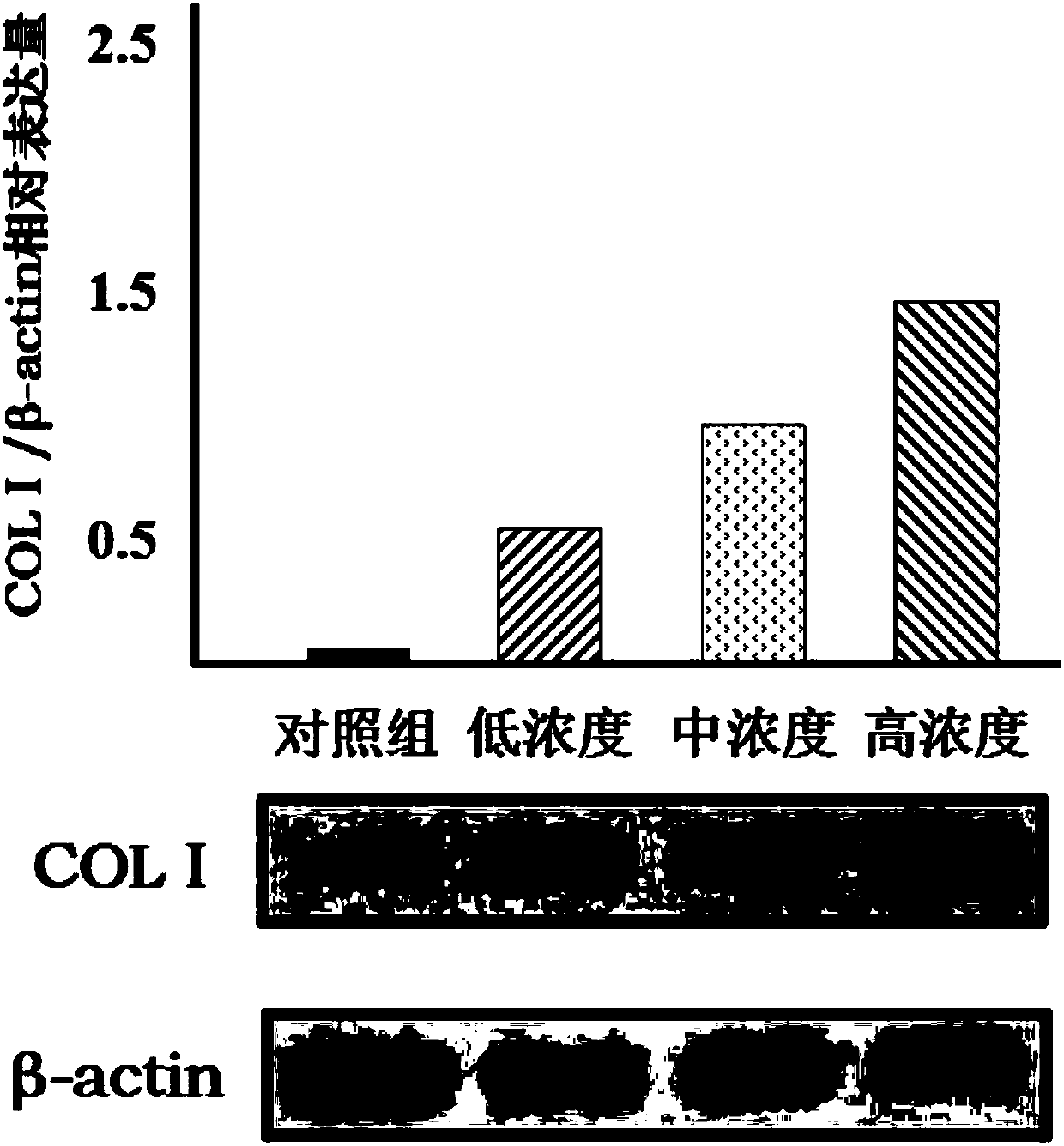
[0020] Example 1 Sargrelate Hydrochloride Impurity I Induces Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells (BMSCs)
[0021] 1. Experimental materials
[0022] Low-sugar DMEM medium (Gibco, USA), fetal bovine serum (Gibco, USA), 0.25% trypsin (containing 0.02% EDTA, Gibco, USA), alizarin red S (Sigma, USA), goat COLⅠ polyclonal antibody ( Santa Cruz, USA). Impurity Ⅰ of sargrelor hydrochloride is self-made, and the HPLC normalized purity is greater than 98%.
[0023] 2. Experimental method
[0024] 1. Isolation and culture of BMSCs
[0025] Take 2 healthy SD rats aged 3 weeks, weighing about 100g, male or female, given an excessive amount of 10% chloral hydrate for intraperitoneal injection to death, soaked in 75% alcohol for 10min, and double-dipped the rats under aseptic conditions. Take out the tibia and femur on the side, and remove the attached tissues. When removing the cartilage tissue at the joint and the bone ends on both sides, pay attention to ...
Embodiment 2
[0047] The preparation of embodiment 2 solid dispersion
[0048] Dissolve sarcogrelate hydrochloride impurity I and povidone K-30 in absolute ethanol according to the following mass ratio to prepare a solution with a mass concentration of sarcogrelate hydrochloride impurity I of 10mg / mL, and stir magnetically at room temperature for 4h (100r / min) , the solvent was removed by rotary evaporation under reduced pressure at 50°C, dried in vacuum for 24 hours, crushed through a No. 5 sieve, and stored in a desiccator for future use.
[0049] Solid dispersion 1: the mass ratio of sarcogrelate hydrochloride impurity I to povidone K-30 is 1:5;
[0050] Solid dispersion 2: the mass ratio of sarcogrelate hydrochloride impurity I to povidone K-30 is 1:4.5;
[0051] Solid dispersion 3: the mass ratio of sarcogrelate hydrochloride impurity I to povidone K-30 is 1:5.5;
[0052] Solid dispersion 4: the mass ratio of sarcogrelate hydrochloride impurity I to povidone K-30 is 1:4;
[0053] So...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com