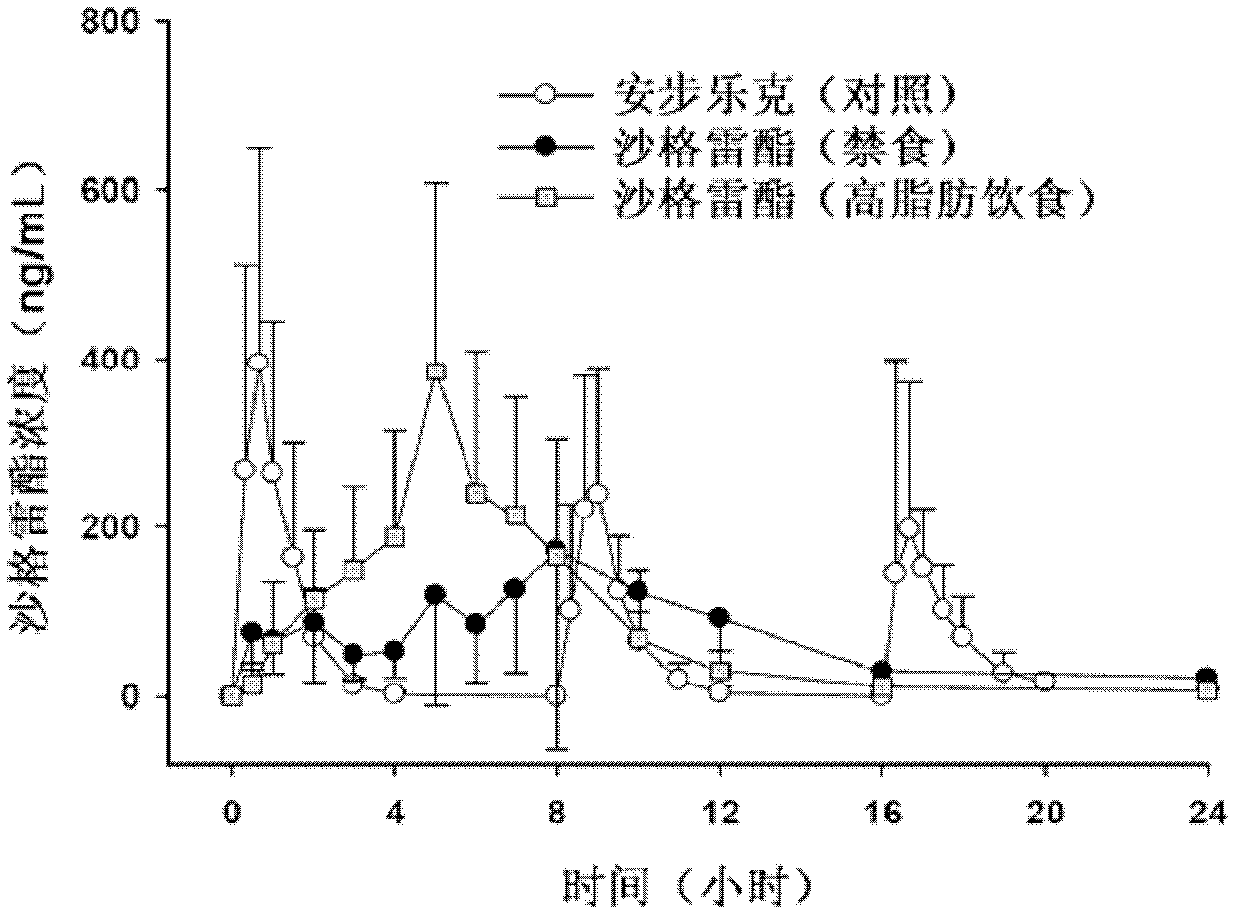
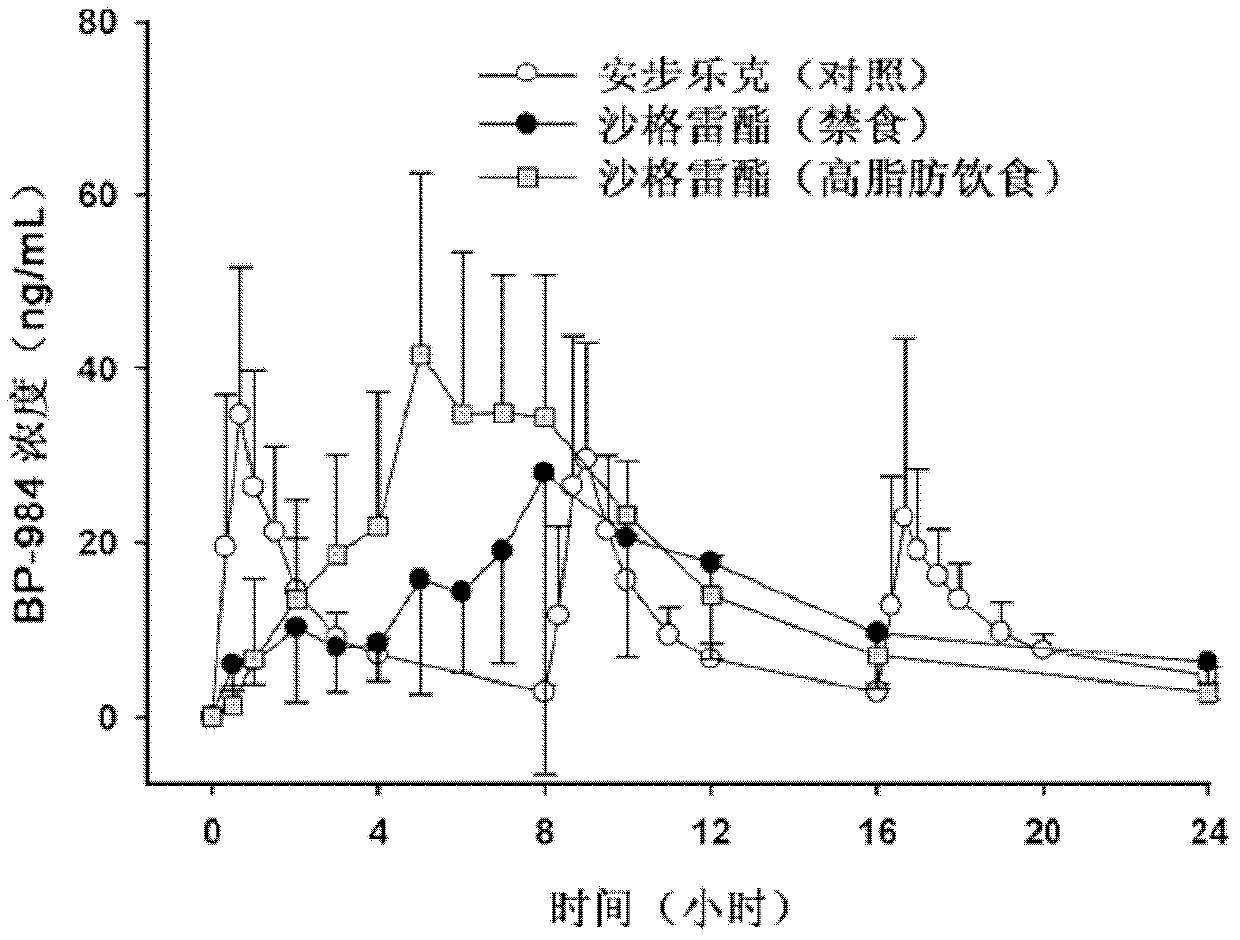
Sarpogrelate hydrochloride sustained release preparation and preparation method thereof
A technology of sarcogrelate hydrochloride and preparations, which is applied in the field of sarcogrelate hydrochloride, can solve the problems of patient burden, foreign body sensation and rupture in the body, and achieve the effects of delaying drug release, preventing vasoconstriction, and antithrombotic vasoconstriction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0019] Examples 1-3 are for comparing the effects of different types of water-soluble polymers on drug release. It is made by dry granulation and tabletting.
[0020] Mix sarcogrelate hydrochloride and water-soluble polymer (hydroxypropyl methylcellulose, or carbo, polyethylene oxide), lactose, hydroxyethylcellulose in a mixer for 5 minutes and then dry granulate (GL -25B, pioneered in Zhangjiagang). Magnesium stearate was added to the prepared granules and mixed for 3 minutes to obtain the final blend. Tablets were compressed on a rotary tablet press (KT-1000) to obtain tablets of 8 to 20 kgf.
[0021] The composition of table 1 embodiment 1~3
[0022] Raw material name
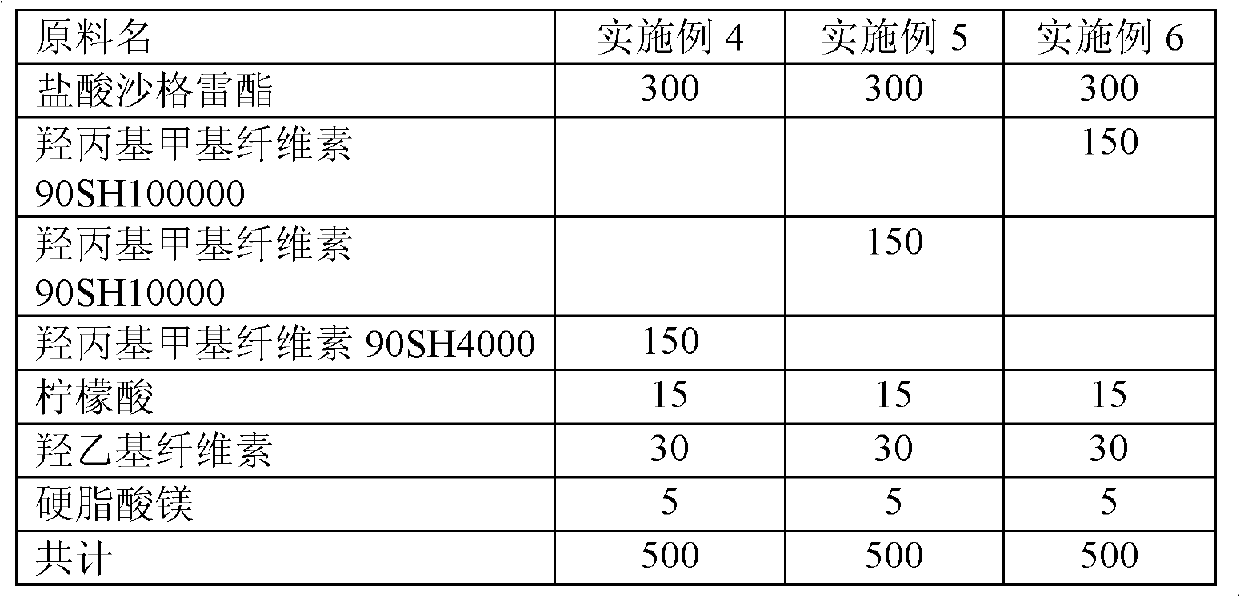
Embodiment 4~6
[0024] Examples 4-6 In order to evaluate the effect of different drug releases, hydrophilic hydroxypropyl methylcellulose, polymers of different viscosities and different grades of displacing agents were used. The manufacturing method is wet granulation.
[0025] First, hydroxypropyl methylcellulose was dissolved in absolute ethanol to obtain a mixed solution. In addition, sarcogrelate hydrochloride, hydroxypropyl methylcellulose, and lactose were rotated and stirred at a high speed. After standing for 5 minutes, a mixed solution was obtained. Then slowly add the previously configured mixture, mix and granulate. The prepared medicine granules are dried for 4 hours under the condition of 60 degrees to obtain the medicine granules with a dry weight below 2%. Finally, after screening, 1 mm pellets were obtained. Then the granules are placed in the mixer, magnesium stearate is added, mixed for 3 minutes, and compressed with a rotary tablet machine to obtain tablets of 8 to 20 kg...
Embodiment 7~8
[0029] Examples 7-8 are comparative formulations made in order to evaluate the effect of the added amount of hydroxypropyl methylcellulose on drug release.
[0030] The manufacturing method is the same as in Examples 4-6.
[0031] The composition of table 3 embodiment 7~8 (unit: mg / T)
[0032]
[0033] Dissolution rate evaluation
[0034] The target dissolution rate of the sustained-release preparation is defined through pharmacokinetic characteristics and literature materials, 10-40% in 4 hours, 40-70% in 10 hours, and more than 80% in 24 hours. Taking Anplag (sargrelate hydrochloride 100mg) produced by YUHAN Company, which is on the market for trial sale, as an example, the dissolution test was carried out together with each example tablet according to the following dissolution test conditions stipulated in Article 1 of the dissolution test method in the general test method of the Korean Pharmacopoeia , compared to the target dissolution rate. At the same time, during ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com