Miniaturization sarpogrelate hydrochloride oral drug-giving preparation
A technology for sarpogrelate hydrochloride and preparations, which is applied in the field of sarpogrelate hydrochloride oral administration preparations, can solve the problems of increased size, difficulty in taking, increased dosage of additives, etc., and achieves good manufacturability, fast dissolution speed, and inhibition of hydrolysis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
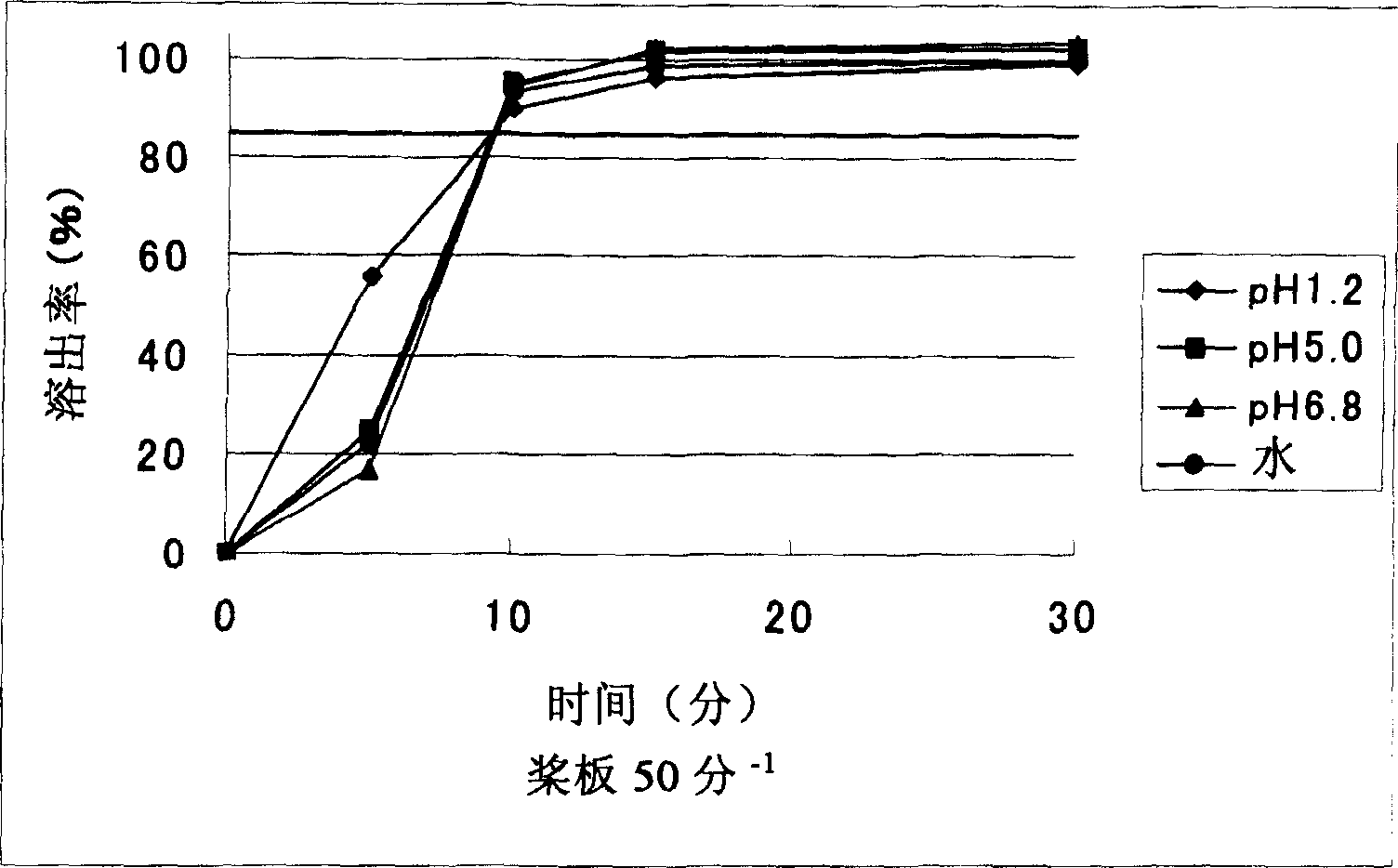
[0083] Example 1: Manufacture of miniaturized sargrelate hydrochloride oral preparation containing carboxymethylcellulose
[0084] (1) 56% by weight of sagrelorate hydrochloride, 19% by weight of crystalline cellulose, 14% by weight of carboxymethyl cellulose (manufactured by NICHIRIN CHEMICAL INDUSTRIES, LTD., NS-300) and 1% by weight of light anhydrous silicon Acid mix. Then, using a high-speed stirring granulator (manufactured by POWREX CORPORATION, a vertical granulator), granulate in an aqueous solution containing 1% by weight of citric acid and 2% by weight of hydroxypropylcellulose, and then dry to obtain granulated particles. 2.4% by weight of magnesium stearate was added to the granules, and compression molding (7.6 mm in diameter) was performed with a rotary tablet machine to obtain uncoated tablets.
[0085] (2) 3% by weight of hydroxypropylmethylcellulose (manufactured by Shin-Etsu Chemical, TC-5RG), 0.6% by weight of titanium oxide, 0.6% by weight of polyethylen...
Embodiment 2
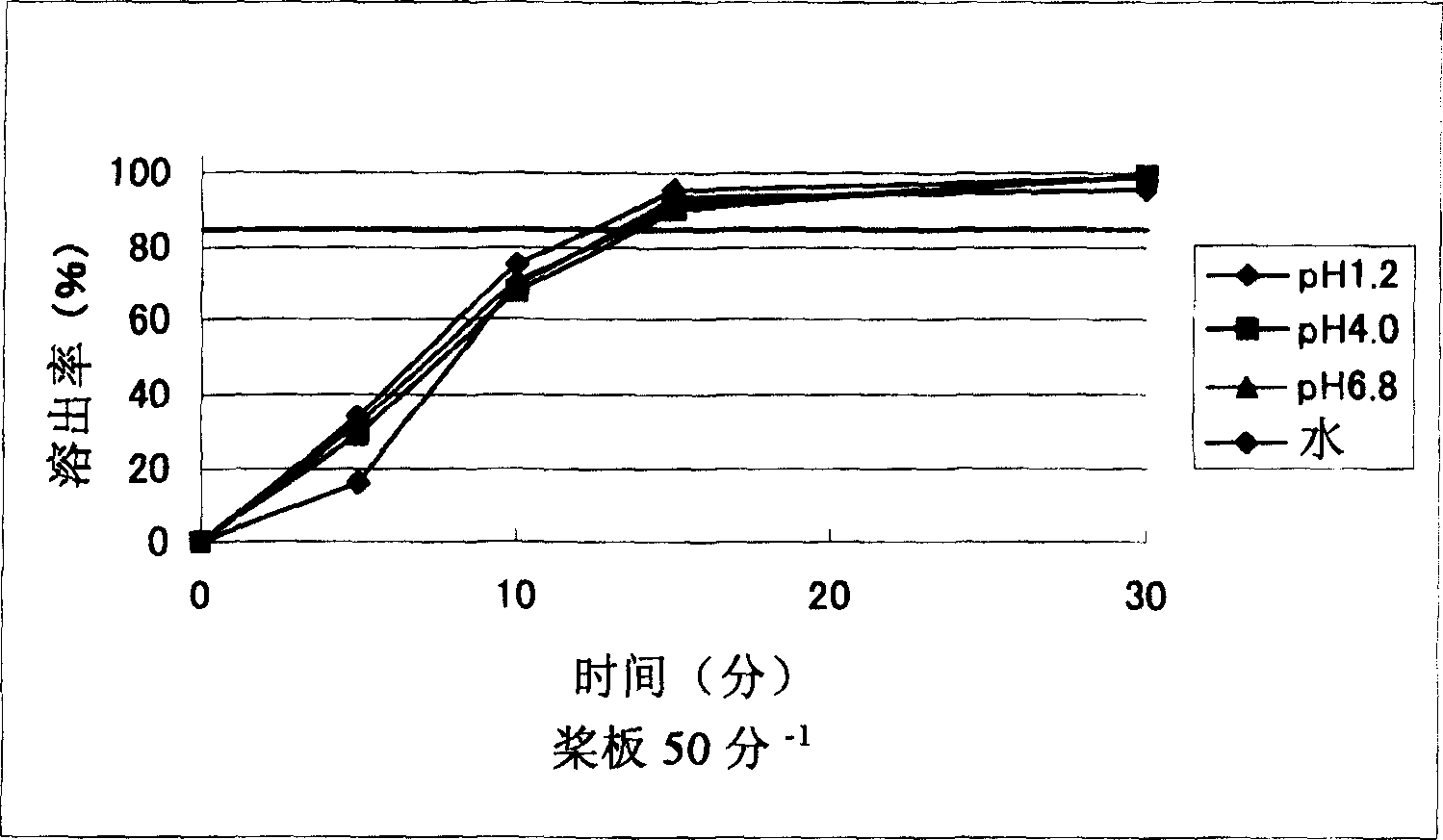
[0092] Example 2: Manufacture of a miniaturized sagrelate hydrochloride oral preparation containing carmellose calcium
[0093] (1) 56% by weight of sagrelorate hydrochloride, 19% by weight of crystalline cellulose, 14% by weight of carmellose calcium (manufactured by NICHIRIN CHEMICAL INDUSTRIES, LTD, ECG505) and 1% by weight of light anhydrous silicic acid were mixed. Then, using a high-speed stirring granulator (manufactured by POWREX CORPORATION, a vertical granulator), granulate in an aqueous solution containing 1% by weight of citric acid and 2% by weight of hydroxypropylcellulose, and then dry to obtain granulated particles. 2.4% by weight of magnesium stearate was added to the granules, and compression molding (7.5 mm in diameter) was performed with a rotary tablet machine to obtain uncoated tablets.
[0094] (2) 3% by weight of hydroxypropylmethylcellulose (manufactured by Shin-Etsu Chemical, TC-5RG), 0.6% by weight of titanium oxide, 0.6% by weight of polyethylene g...
Embodiment 3
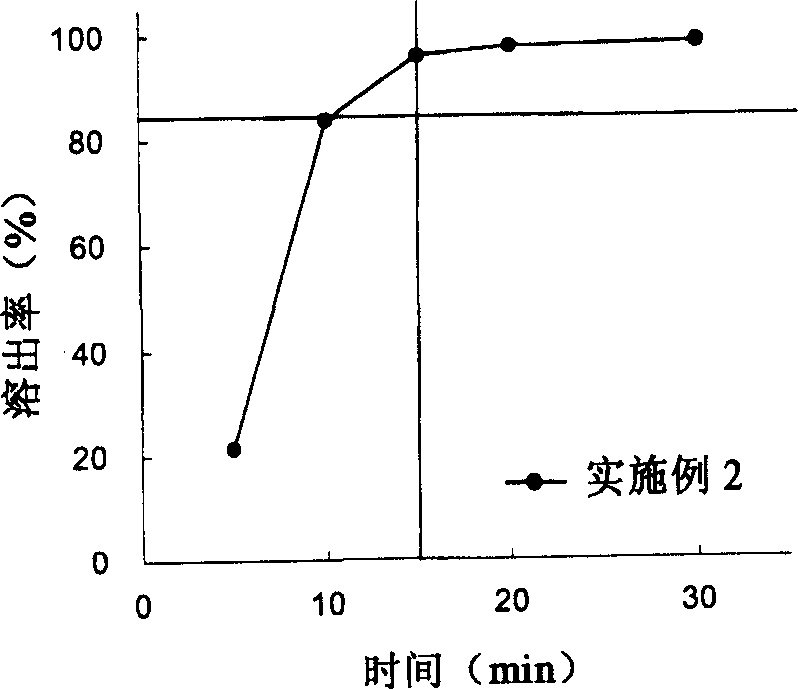
[0096] Embodiment 3: the manufacture (stirring granulation) of the miniaturized sarcogrelate hydrochloride oral administration preparation containing sodium carboxymethyl starch
[0097] (1) 56% by weight of sagrelorate hydrochloride, 23% by weight of crystalline cellulose, 10% by weight of sodium carboxymethyl starch (manufactured by DMV, Primogel), and 1% by weight of light anhydrous silicic acid were mixed. Then, using a high-speed stirring granulator (manufactured by POWREX CORPORATION, a vertical granulator), granulate in an aqueous solution containing 1% by weight of citric acid and 2% by weight of hydroxypropylcellulose, and then dry to obtain granulated particles. 2.4% by weight of magnesium stearate was added to the granules, and compression molding (7.5 mm in diameter) was performed with a rotary tablet machine to obtain uncoated tablets.
[0098] (2) 3% by weight of hydroxypropylmethylcellulose (manufactured by Shin-Etsu Chemical, TC-5RG), 0.6% by weight of titaniu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com