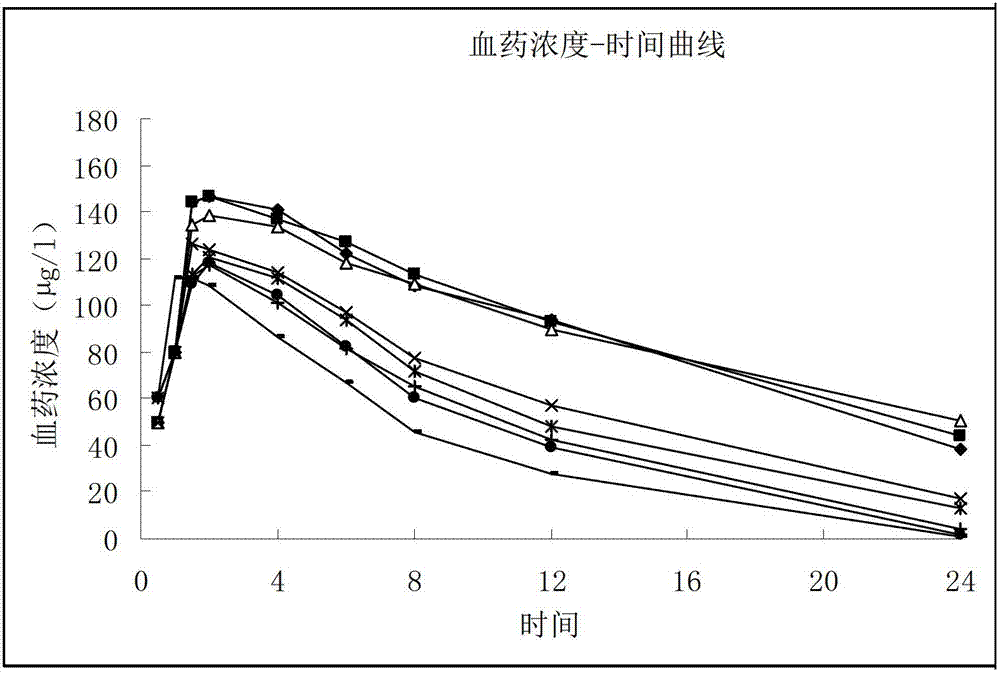
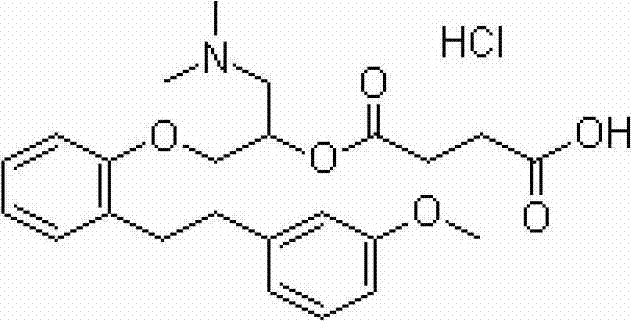
Sarpogrelate hydrochloride lipidosome solid preparation
A technology of sarcogrelate hydrochloride lipid and solid preparations, which is applied in the field of pharmaceutical preparations, can solve the problems of poor production controllability, unsatisfactory reproducibility of sustained-release effects of preparations, low bioavailability of sarcogrelate hydrochloride, etc., and achieve Improve bioavailability, improve drug compliance, and increase retention time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] According to another aspect of the present invention, the preparation method of above-mentioned sarcogrelate hydrochloride liposome is provided, and the method comprises the following steps:
[0062] (a) Dissolve dilauroylphosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol and cholesterol in an organic solvent, mix well and sonicate for 30 minutes to prepare blank liposomes;
[0063] (b) Dissolving sarcogrelate hydrochloride in blank liposome suspension, incubating at 59° C. for 30-40 minutes, ultrasonication for 30-40 minutes, filtering through a 0.45 μm microporous membrane, and freeze-drying to obtain a solid liposome.
[0064] In a preferred embodiment of the method for preparing sarcogrelate hydrochloride liposomes according to the present invention, in step (a), the organic solvent is selected from the group consisting of ethanol, methanol, acetone, ether, chloroform, n-hexane, di One of methyl chloride, preferably ethanol solvent.
[0065] In a p...
Embodiment 1
[0091] The preparation of embodiment 1 sarcogrelate hydrochloride liposome sheet
[0092] The raw materials used are as follows:
[0093]
[0094] The preparation process is as follows:
[0095] (1) Dissolve 100g of dilauroylphosphatidylcholine, 100g of phosphatidylethanolamine, 100g of phosphatidylglycerol and 100g of cholesterol in 800ml of ethanol, mix well and sonicate for 30 minutes to make a blank liposome;
[0096] (2) Dissolve 100 g of sarcogrelate hydrochloride in the blank liposome suspension, incubate at 59° C. for 30 minutes and sonicate, filter through a 0.45 μm microporous membrane, and freeze-dry to obtain solid liposomes;
[0097] (3) Sieve the sarcogrelate hydrochloride liposome solid through an 80-mesh sieve, then mix it with 50 g of microcrystalline cellulose and 40 g of low-substituted hydroxypropyl cellulose, and mix evenly through a sieve, then add 5% povidone K30 in 50% ethanol solution Wet 100ml to prepare soft material, pass through a 24 mesh siev...
Embodiment 2
[0100] The preparation of embodiment 2 sarcogrelate hydrochloride liposome sheet
[0101] The raw materials used are as follows:
[0102]
[0103] The preparation process is as follows:
[0104] (1) Dissolve 240g of dilauroylphosphatidylcholine, 100g of phosphatidylethanolamine, 150g of phosphatidylglycerol and 150g of cholesterol in 1000ml of ethanol, mix well and sonicate for 30 minutes to make a blank liposome;
[0105] (2) Dissolve 100 g of sarcogrelate hydrochloride in the blank liposome suspension, incubate at 59° C. for 30 minutes and sonicate, filter through a 0.45 μm microporous membrane, and freeze-dry to obtain solid liposomes;
[0106] (3) Sieve the sarcogrelate hydrochloride liposome solid through an 80-mesh sieve, then mix it with 60 g of microcrystalline cellulose and 50 g of low-substituted hydroxypropyl cellulose, sieve and mix evenly, and add 5% povidone K30 in 50% ethanol solution Wet 120ml to prepare soft material, granulate through 24 mesh sieve, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com