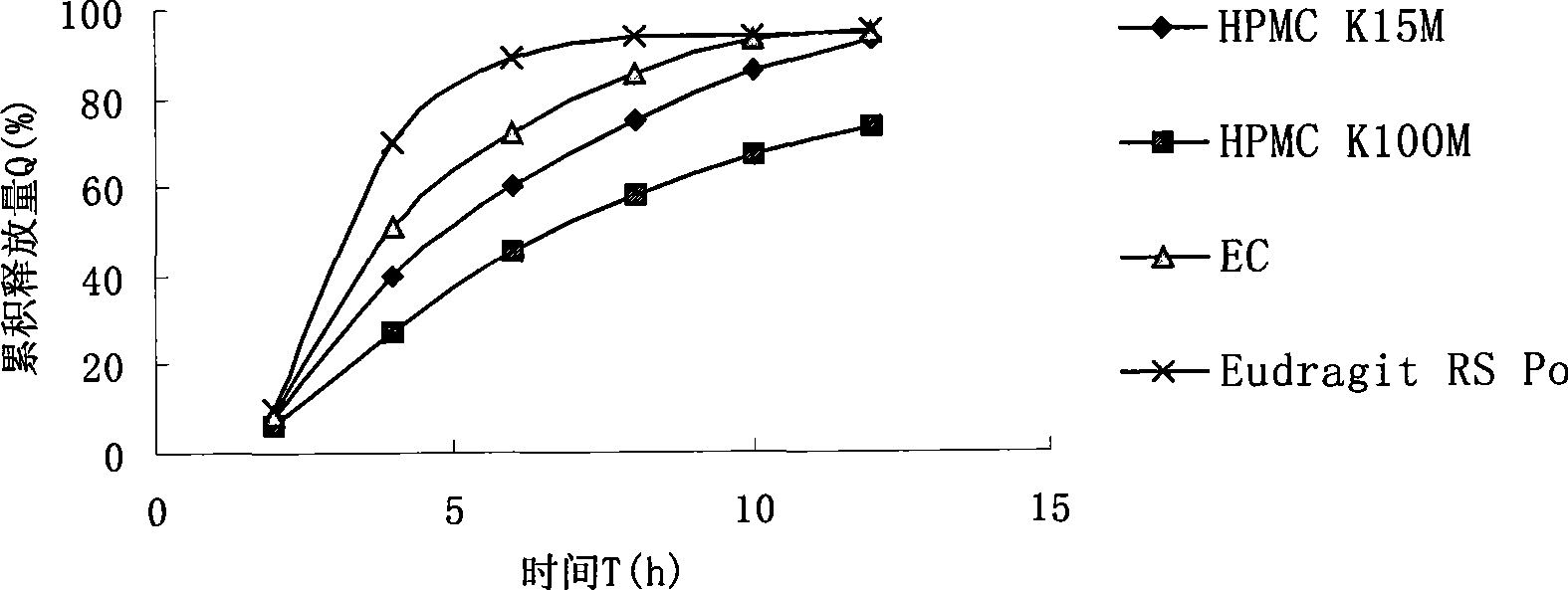
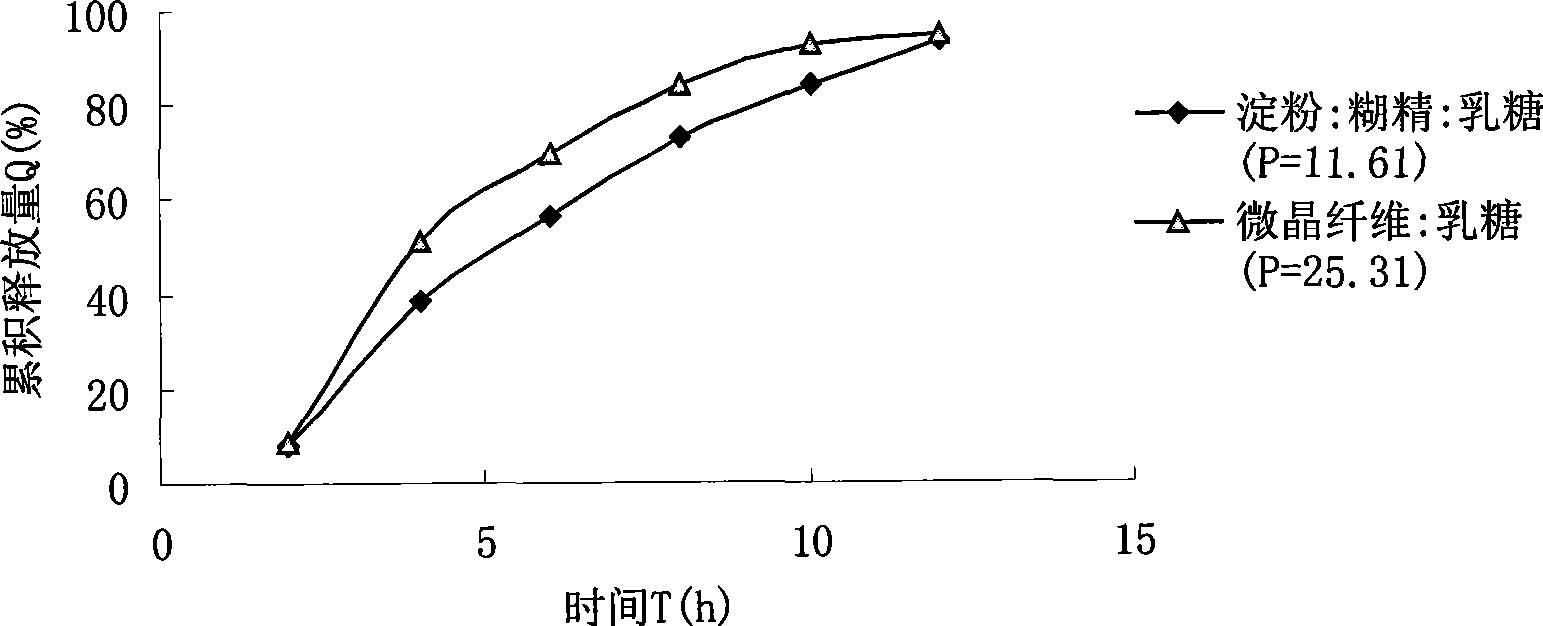
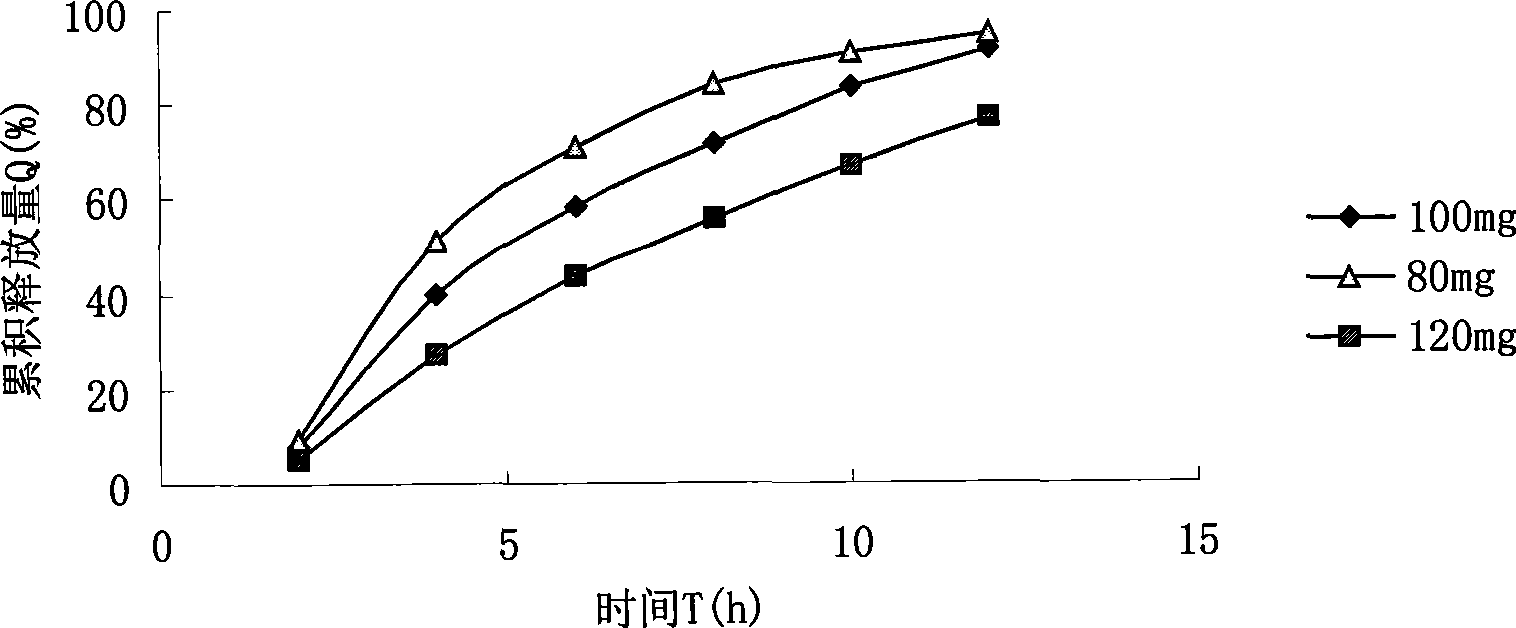
Novel dosage form of sinomenine medicament or hydrochlorate thereof and preparation technique thereof
A technology of sinomenine and hydrochloride, which is applied in the field of sinomenine or its hydrochloride enteric-coated controlled-release tablet and its preparation technology, can solve the toxic and side effects, low bioavailability, and cannot meet clinical needs And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220] Example 1: Preparation of matrix-type enteric-coated controlled-release tablets
[0221] 1. Tablet core composition
[0222] Sinomenine 60.0kg Hypromellose 100.0kg
[0223] Starch 40.0kg Dextrin 60.0kg
[0224] Lactose 90.0kg 70% ethanol solution 40L
[0225] Magnesium Stearate 3.5kg
[0226] 2. Enteric coating ingredients
[0227] Kalekang Yakeyi Enteric Coating Prepowder 52.5kg Purified Water 210L;
[0228] Pass the sinomenine raw material through an 80-mesh sieve, pass each auxiliary material through an 80-mesh sieve, mix according to the proportion, add 70% ethanol solution, make a soft material, granulate with a 20-mesh sieve, dry at 40°C for 2 hours, granulate, add 1 % magnesium stearate, mixed evenly, and pressed into tablets to obtain the skeleton tablet core; weighed the prescribed amount of Kalecon Yakeyi enteric-coated pre-coated powder, dispersed in purified water and stirred evenly, and prepared into enteric-coated prepowder with a solid content of 20%...
Embodiment 2
[0229] Example 2: Preparation of film-controlled enteric-coated controlled-release tablets
[0230] 1. Tablet core composition
[0231] Sinomenine 60.0kg Ethylcellulose 52.1kg
[0232] Microcrystalline cellulose 39.1kg Lactose 198.8 parts by weight
[0233] 3% Hypromellose Aqueous Solution 40L Magnesium Stearate 3.5kg
[0234] 2. Enteric-coated film-controlled ingredients
[0235] Acrylic RS 10.16kg Acrylic RL 1.84kg
[0236] Hypromellose Phthalate 3.19kg Diethyl Phthalate 6.0L
[0237] Talc powder 3kg 80% ethanol 150L;
[0238] Pass the sinomenine raw material through an 80-mesh sieve, pass each auxiliary material through an 80-mesh sieve, mix according to the proportion, add 3% hypromellose aqueous solution, make soft material, granulate with a 20-mesh sieve, dry at 40°C for 2 hours, and dry granules, add 1% magnesium stearate, mix well, and press into tablets to get the skeleton tablet core; weigh the prescribed amount of enteric-coated film-controlled coating ingredien...
Embodiment 3
[0239] Example 3: Preparation of matrix-type enteric-coated controlled-release tablets
[0240] 1. Tablet core composition
[0241] Sinomenine 120kg Hypromellose 100kg
[0242] Starch 10kg Dextrin 60kg
[0243] Lactose 60kg 70% ethanol solution 50L
[0245] 2. Enteric coating ingredients
[0246] Kalekon Yakeyi Enteric Coating Prepowder 60kg Purified Water 290L;
[0247] Pass the sinomenine raw material through an 80-mesh sieve, pass each auxiliary material through an 80-mesh sieve, mix according to the proportion, add 70% ethanol solution, make a soft material, granulate with a 20-mesh sieve, dry at 40°C for 2 hours, granulate, add 1 % magnesium stearate, mixed evenly, and pressed into tablets to obtain the skeleton tablet core; weighed the prescribed amount of Kalecon Yakeyi enteric-coated pre-coated powder, dispersed in purified water and stirred evenly, and prepared into enteric-coated prepowder with a solid content of 20%. Dissolve th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com